A kind of preparation method of anti-human thymus stromal lymphopoietin (tslp) monoclonal antibody concentrated solution
A monoclonal antibody and lymphocyte technology, applied in the biological field, can solve problems such as process failure, concentration polarization out of control, and reduced sliding performance of the drug-filled syringe, and achieve good clinical results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0058] The application provides a method for preparing an anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody concentrated solution, comprising the following steps:
[0059] Step 1: Concentrating the solution containing anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody to obtain the first concentrated sample;
[0060] Step 2: replacing the first concentrated sample with a buffer solution to obtain a replacement concentrated solution;
[0061] Step 3: mixing the concentrated replacement solution and the mother liquor of the amino acid protecting agent to obtain a mixed solution, and performing ultrafiltration and concentration on the mixed solution to obtain a second concentrated sample;
[0062] The anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody comprises three heavy chain complementarity determining regions (CDR-H1, CDR-H2 and CDR-H3) and three light chain complementarity determining regions (CDR-L1, CDR-L2 and CDR-L3), whe...
Embodiment 1
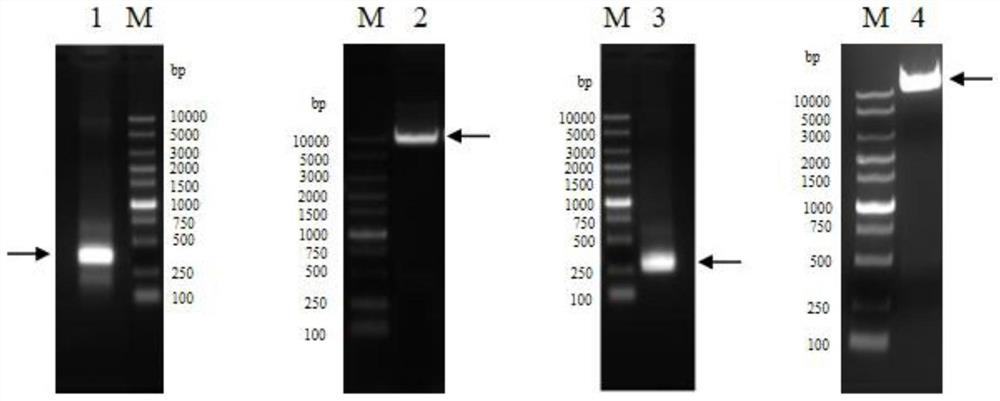
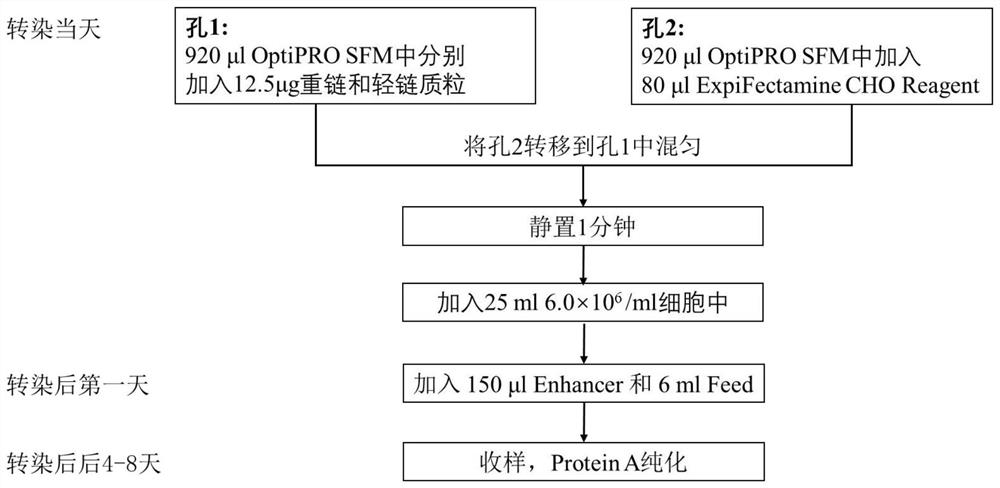
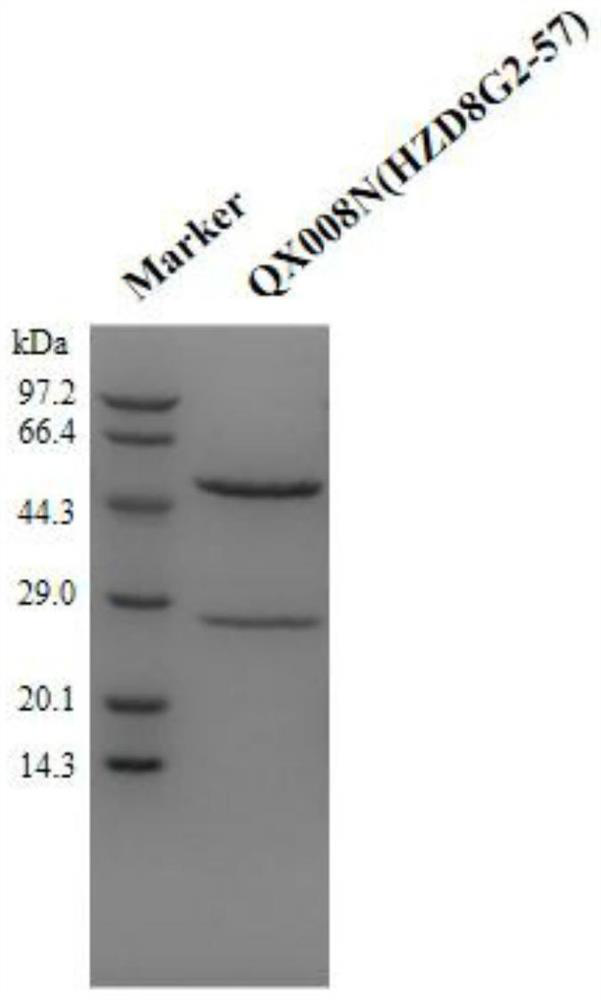
[0151] Embodiment 1 Preparation of anti-human TSLP monoclonal antibody QX008N
[0152] Purchasing human thymic stromal lymphopoietin (hTSLP) from Shanghai Nearshore Technology Co., Ltd. for immunization of New Zealand rabbits, using B cell cloning technology to obtain antigen-binding specific antibody clones, and then screening for binding to human TSLP and having human TSLP inhibitory activity of monoclonal antibodies. The cell supernatant was detected by Binding ELISA and Blocking ELISA, and the target clone was selected. The above immunization and screening processes are entrusted to commercial companies.
[0153]Seven clones were selected for recombinant expression and sequenced. It has been determined that 8G2 has the best cell neutralizing activity. Therefore, the 8G2 clone was humanized. Use NCBI IgBlast to carry out homology comparison of human IgG germline sequence (Germline), select IGHV3-66*01 as the heavy chain CDR transplantation template, and clone the CDR re...
Embodiment 2
[0158] The mensuration of embodiment 2 equilibrium dissociation constant (KD)
[0159] The affinity between QX008N(HZD8G2-57) and human TSLP was detected by Biacore T200, and all processes were carried out at 25°C. Using a commercial Protein A chip, an appropriate amount of antibody was immobilized by the capture method, so that the Rmax was around 50RU, and the capture flow rate was 10 μl / min. The antigen was serially diluted, the flow rate of the instrument was switched to 30 μl / min, and the concentration flowed through the reference channel and the channel of the immobilized antibody in order of concentration from low to high, and the buffer was used as a negative control. After each association and dissociation, the chip was regenerated with pH 1.5 glycine. Use the instrument's own analysis software to select the 1:1 binding model in the Kinetics option for fitting, and calculate the antibody's association rate constant ka, dissociation rate constant kd, and dissociation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com