Preparation method of trifluoromethylated 2,4-quinolinedione compound
A technology of trifluoromethylation and quinoline dione, applied in the direction of organic chemistry, etc., can solve problems such as limited amplification application, and achieve the effect of reducing side reactions and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The present invention will be further described in detail below in conjunction with the embodiments, so that those skilled in the art can implement it with reference to the description.
[0018] It should be noted that the experimental methods described in the following embodiments, unless otherwise specified, are conventional methods, and the reagents and materials, unless otherwise specified, can be obtained from commercial sources;
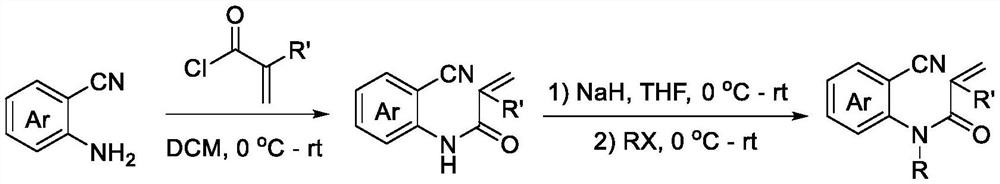
[0019] According to the method reported in the existing literature (Organic & Biomolecular Chemistry, 2015, 13, 5376-5380), the reaction raw materials required for the technology of the present invention were synthesized. Concrete synthetic scheme is as follows:
[0020]
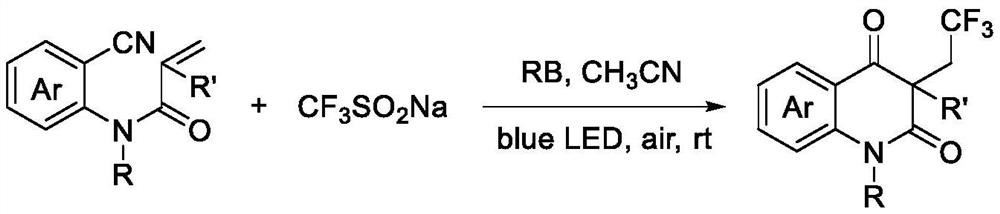
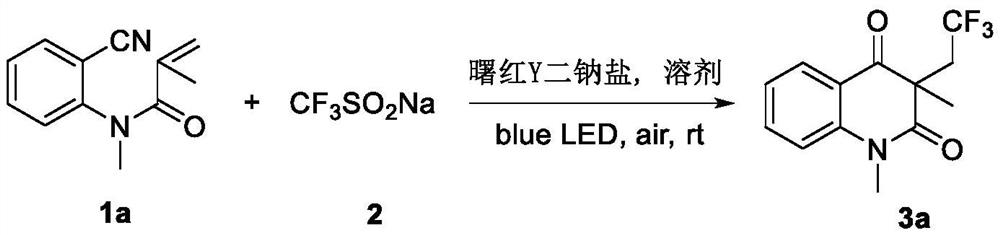
[0021] The preparation method of trifluoromethylated 2,4-quinolinediones, comprising:
[0022] Add N-o-cyanoaryl acrylamide compound, sodium trifluoromethyl sulfinate, photocatalyst and solvent successively into the reactor, then stir the reaction under air atmosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com