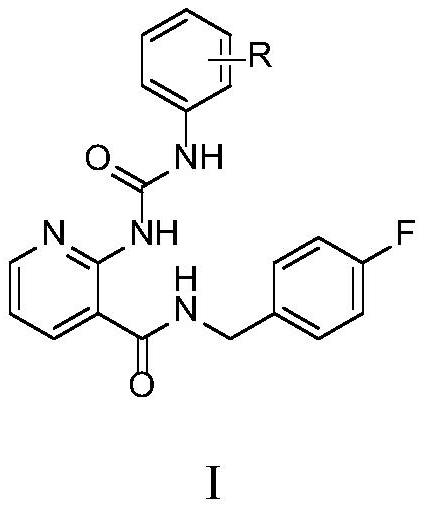
2-arylureido-N-(4-fluorobenzyl)nicotinamide compound and application thereof
A technology of nicotinamide and fluorobenzyl, which is applied in the application field of anti-influenza virus drugs, and achieves the effects of stable yield, extensive therapeutic effect, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of 2-(3-phenylureido)-N-(4-fluorobenzyl)nicotinamide
[0037]
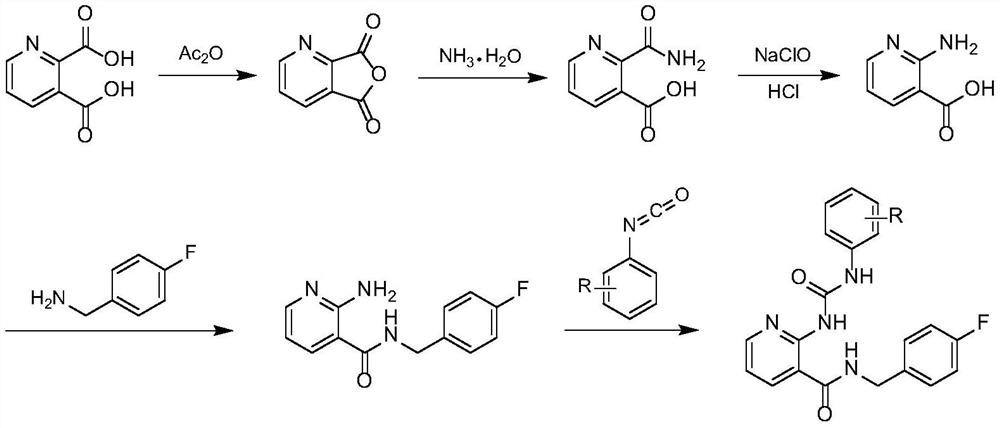
[0038] Step A: Preparation of 2,3-pyridinedicarboxylic anhydride
[0039] Put 16.7g (100mmol) of 2,3-pyridinedicarboxylic acid in a 100mL eggplant-shaped bottle, add 20.0mL of acetic anhydride, heat up and reflux for 30min, cool, crystallize, filter with suction, and dry to obtain 2,3-pyridinedicarboxylic anhydride Pale yellow solid 12.4g, yield 83.2%, m.p.: 132.6-133.8°C. (Literature (GRIBBLE G, FLETCHER G, KETCHA D, et al. Metalated heterocycles in the synthesis of ellipticine analogues: A new route to the 10H-pyrido[2,3-b]carbazole ring system. Journal of Organic Chemistry, 1989, 54( 14): 3264-3269.) Value: 133-134°C).
[0040] Step B: Preparation of 2-carbamoylpyridine-3-carboxylic acid
[0041]Put 13.6g (200mmol) of 25% ammonia water in a 100mL eggplant-shaped bottle, and slowly add 7.46g (50mmol) of 2,3-pyridine dicarboxylic anhydride in an ice-water bath, stir for 2.5h, t...
Embodiment 2
[0049] Example 2: Preparation of 2-[3-(4-methylphenyl)ureido]-N-(4-fluorobenzyl)nicotinamide
[0050]
[0051] According to the preparation method of Example 1, 0.68 g of white solid was obtained with a yield of 45.0%. m.p.:206.1-208.0℃;IR:(KBr,cm -1 ) 3336, 3063, 2906, 1684, 1615, 1544, 1507, 1435, 1405, 737; 1 H-NMR (400MHz, CDCl 3 ):δ2.32(s,3H,CH 3 ), 4.61(d,2H,ArCH 2 NH),6.59(brs,1H,NH),6.89(dd,1H,Ar-H,J 1 =7.6Hz,J 2 =4.8Hz), 7.05(t,2H,Ar-H,J=8.8Hz),7.13(d,2H,Ar-H,J=8.4Hz),7.34(dd,2H,Ar-H,J 1 =8.4Hz,J 2 =6.4Hz), 7.46(d,2H,Ar-H,J=8.4Hz),7.80(dd,1H,Ar-H,J 1 =7.6Hz,J 2 =1.6Hz), 8.34(dd,1H,Ar-H,J 1 =4.8Hz,J 2 =1.2Hz), 10.38(s,1H,NH),11.66(s,1H,NH); ESI-MS(m / z):379.1([M+H] + ).
Embodiment 3
[0052] Example 3: Preparation of 2-[3-(4-methoxyphenyl)ureido]-N-(4-fluorobenzyl)nicotinamide
[0053]
[0054] According to the preparation method of Example 1, 0.52 g of white solid was obtained with a yield of 33.0%. m.p.:199.5-200.3℃;IR:(KBr,cm -1 ) 3363, 3047, 2837, 1673, 1629, 1541, 1512, 1487, 1417, 740; 1 H-NMR (400MHz, CDCl 3 ):δ3.80(s,3H,OCH 3 ),4.60(d,2H,ArCH 2 NH),6.58(brs,1H,NH),6.86-6.91(m,3H,Ar-H),7.05(t,2H,Ar-H,J=8.8Hz),7.34(dd,2H,Ar-H ,J 1 =8.4Hz,J 2 =5.6Hz), 7.48(d,2H,Ar-H,J=8.8Hz),7.80(dd,1H,Ar-H,J 1 =7.6Hz,J 2 =1.2Hz), 8.33(dd,1H,Ar-H,J 1 =5.2Hz,J 2 =1.6Hz), 10.38(s,1H,NH),11.57(s,1H,NH); ESI-MS(m / z):395.2([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com