Atropine alkaloid hapten and artificial antigen as well as preparation methods and application thereof
An artificial antigen and atropine technology, applied in biological testing, chemical instruments and methods, material inspection products, etc., can solve the problems of not being able to adapt to multiple types of preliminary screening, rapid screening, high professional requirements, and complicated pretreatment procedures , to achieve the effect of good application prospect, high sensitivity and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1, preparation and characterization of atropine alkaloid hapten
[0044] One, the preparation of atropine alkaloid hapten
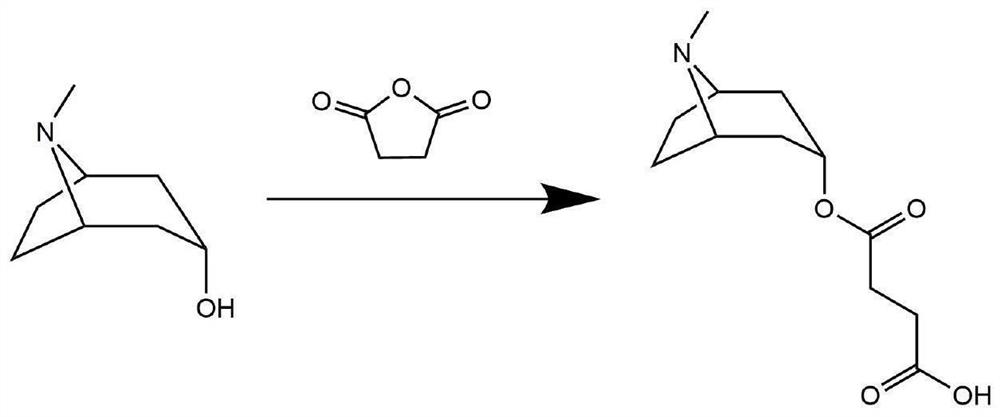
[0045] The preparation of atropine alkaloid hapten shown in formula I, reaction equation is as figure 1 shown.
[0046] The first step: the reaction uses pyridine as a solvent, mixes 1eq tropinol standard product with 1.1eq succinic anhydride, dissolves in a 5mL round bottom flask, installs a reflux device, places in a 70°C oil bath, and stirs magnetically for 24h.
[0047] Step 2: Add 0.1eq succinic anhydride to the reaction solution, stir at 600r / min for 6h, remove most of the pyridine by nitrogen blowing in the fume hood, let it stand for natural volatilization, and form brown crystals without purification.
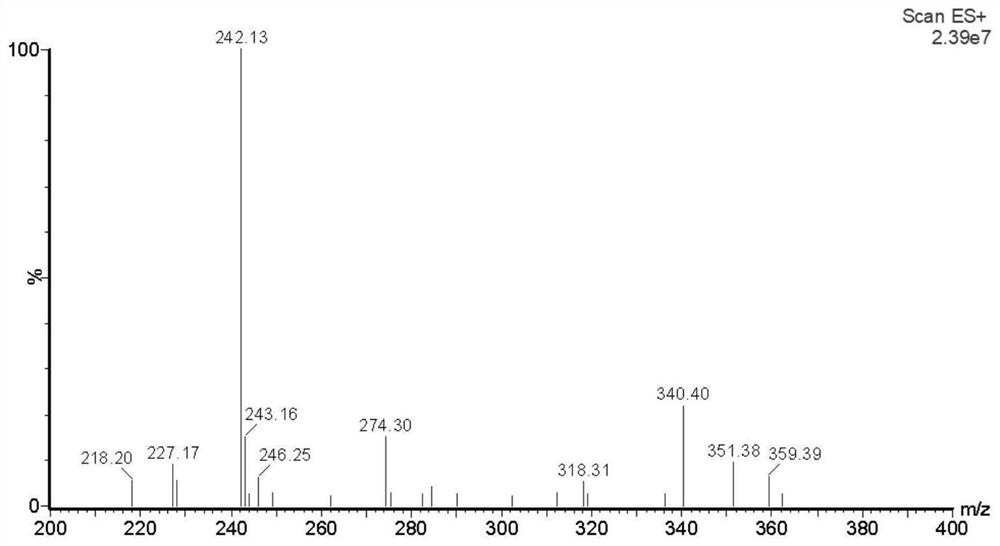
[0048] 2. Characterization of atropine alkaloid hapten
[0049] Mass spectrometry identification: mass spectrometry identification result of atropine alkaloid hapten shown in formula I: MS m / z[M+H] + Theoretical value: 242.28; ...
Embodiment 2
[0050] Example 2, Preparation and Characterization of Atropine Alkaloid Artificial Antigen
[0051] In the preparation methods of the immunogen and the coating source, the difference lies in the type of carrier protein used. The carrier protein of the immunogen is mainly BSA and KLH, the carrier protein of the coating source is mainly OVA, and the coupling method used is the active ester method.
[0052] 1. Synthesis and identification of atropine alkaloid artificial antigen
[0053] 1. Preparation of atropine alkaloid artificial antigen
[0054] (1) Calculate based on the hapten and BSA feeding ratio of 1:100, then accurately weigh 7.3mg of hapten, 8.6mg of EDC, and 5.2mg of NHS according to the molar mass ratio of hapten:EDC:NHS=2:3:3 and dissolve them respectively In 1mL DMF, put it on a magnetic stirrer and activate it for 16h, which is the solution I.
[0055] (2) Dissolve 20 mg of KLH, BSA, and OVA in 10 mL of PBS respectively, pre-cool in a -20°C refrigerator, and the...
Embodiment 3
[0061] Embodiment 3, preparation of atropine alkaloid monoclonal antibody
[0062] The TRO-HS-BSA and TRO-HS-KLH artificial antigens prepared in Example 2 were used to immunize 8 BALB / c female mice aged 6-8 weeks respectively. Each immunogen was diluted to 1 mg / mL with 0.01 M PBS, and emulsified with an equal amount of Freund's adjuvant to form a water-in-oil structure. Except Freund's complete adjuvant was used for the first immunization, Freund's incomplete adjuvant was used for the rest of the booster immunization, and the immunization dose was 100 μg per mouse, and multi-point intradermal injection was used on the back of the neck. After 4 weeks, booster immunization was carried out and immunization was given once every 3 weeks, for a total of 2 additional immunizations, which were changed to multi-point subcutaneous injections on the back of the neck. 7-10 days after the two immunizations, the orbital blood of each mouse was centrifuged to take the supernatant for determ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com