Polyaryl compound in purslane and extraction and separation method thereof
A polyaryl compound and separation method technology, which is applied in the directions of food extraction, ether separation/purification, food ingredients containing natural extracts, etc., can solve the problems of low structural novelty and the like, and achieves environmentally friendly process methods, simple and fast operation methods. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
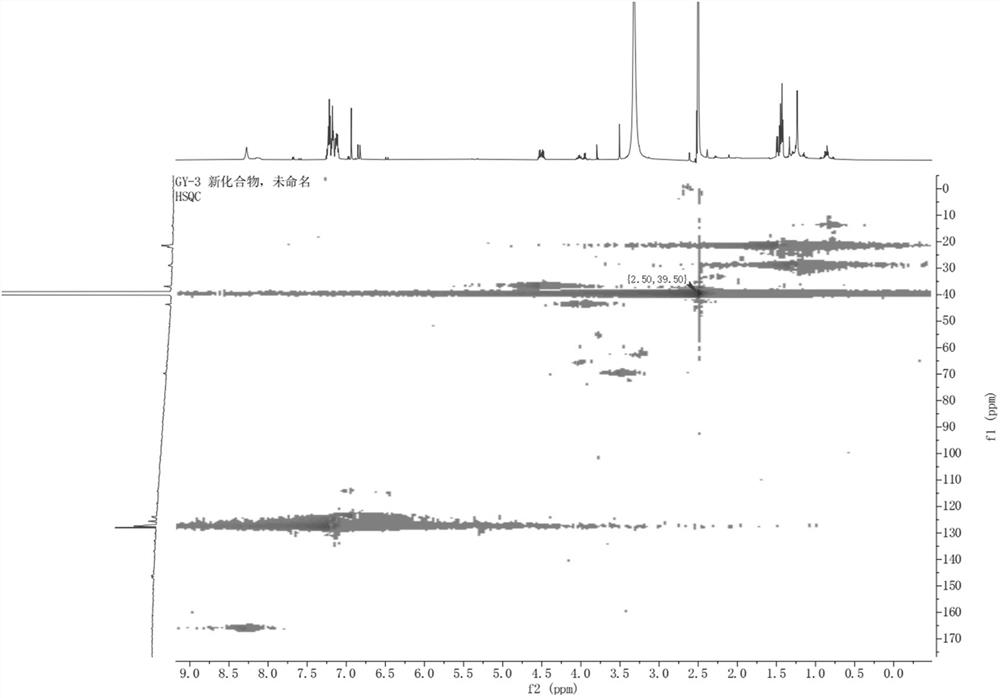
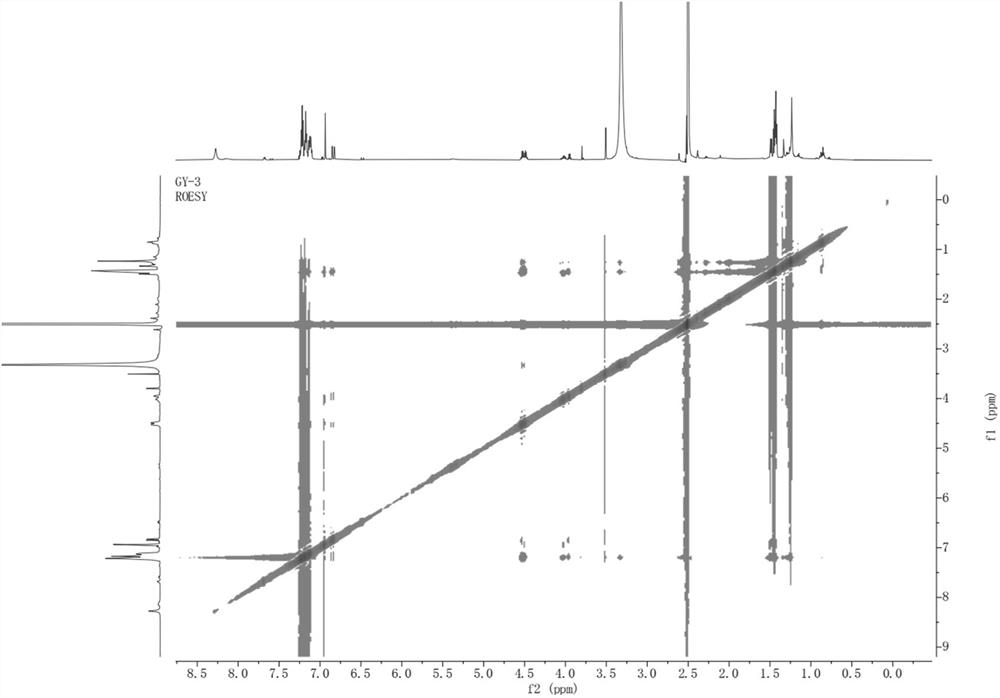
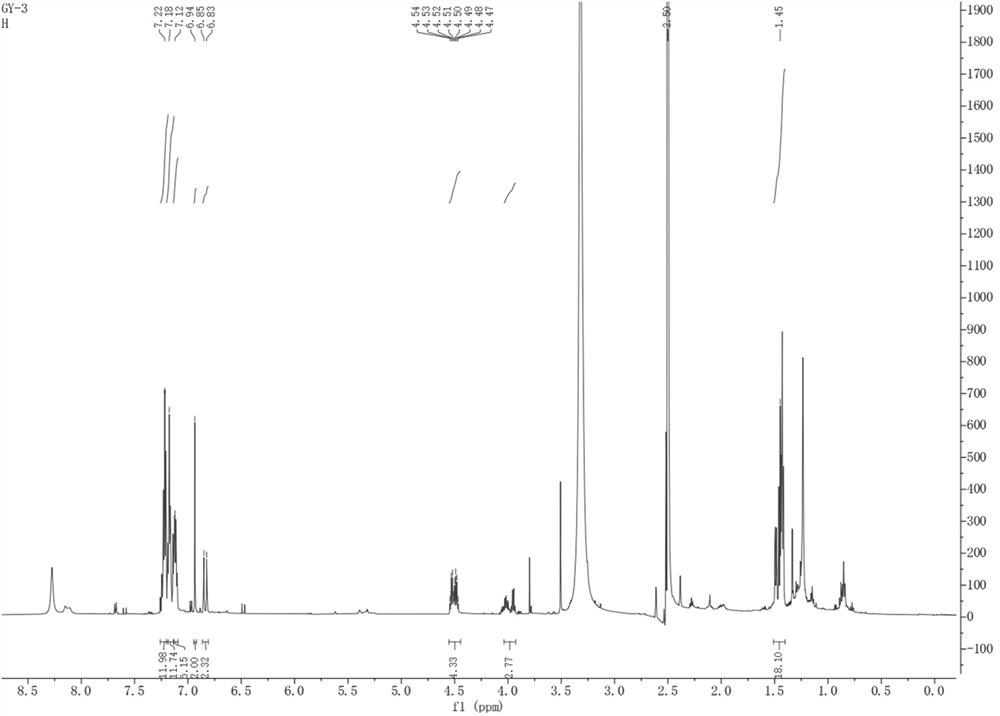
[0035] The present invention provides polyarylate compound, molecular formula is C 60 h 58 O is named 1,1'-oxybis(2-((R)-1-phenylethyl)-4,5-bis((S)-1-phenylethyl)phenyl), and its chemical structure is:
[0036] .
[0037] The polyarylate is named 1,1'-oxybis(2-((R)-1-phenylethyl)-4,5-bis((S)-1-phenylethyl)phenyl) according to the structure, and Table 1 is the NMR data of polyarylates: 1 H-NMR with 13 C-NMR in DMSO.
[0038] Table 1: NMR data of the compound 1,1'-oxybis(2-((R)-1-phenylethyl)-4,5-bis((S)-1-phenylethyl)phenyl) of the present invention
[0039] .
[0040]Structural identification and deduction of the polyaryl compound 1,1'-oxybis(2-((R)-1-phenylethyl)-4,5-bis((S)-1-phenylethyl)phenyl) of the present invention.
[0041] 1,1'-oxybis(2-((R)-1-phenylethyl)-4,5-bis((S)-1-phenylethyl)phenyl): The compound is a white powder, easily soluble in methanol. UHPLC-ESI-Q-TOF-MS gave m / z: 795.4558[M-H] + (C 60 h 59 o + , the calculated value is 795.4561) of the q...
Embodiment 2
[0050] Example 2 Neuroprotective effect of polyaryl compounds of the present invention.
[0051] 1 main material.
[0052] 1.1 Drugs and reagents: The new alkaloid compounds used in the experiment were prepared by the above method, with a purity of 90-99%, weighed accurately, and diluted with DMSO to the required solutions for the following dosage groups. DMEM high-glucose medium, fetal bovine serum (Hyclone Company, USA); penicillin, streptomycin (Hangzhou Sijiqing Company), phosphate buffer saline (PBS), (Wuhan Boster Co., Ltd.), ROS detection kit (Haimen Bi Yuntian Reagent Company).
[0053] 1.2 Cell lines: Human neuroblastoma cell lines (SH-SY5Y, IMR-32) (Shanghai Cell, Chinese Academy of Sciences).
[0054] 1.3 Grouping: divided into control group, H 2 o 2 Damage model group and experimental group.
[0055] 2 Experimental methods.
[0056] 2.1 Cell culture, DMEM high-glucose medium, add 10% fetal bovine serum, 1% antibiotics (100U / mL penicillin and 100μg / mL streptom...
Embodiment 3
[0070] Example 3 Anticholinesterase effect of compounds of the present invention.
[0071] 1. Main materials.
[0072] 1.1. Drugs and reagents: The polyarylates used in the experiment were prepared by the above method with a purity of 90-99%. Sodium dihydrogen phosphate, disodium hydrogen phosphate (Sinopharm Chemical Reagent Co., Ltd.), physostigmine (Hanxiang Biotechnology ), phosphorus 5,5'-dithiobis(2-nitrobenzoic acid) (Dithiobisnitrobenzoic acid, DTNB, Shanghai Jinsui Biotechnology Co., Ltd.), acetylcholinesterase (AChE) and iodide thioacetylcholine (Acetylthiocholine iodide , ATCI, Dalian Meilun Biotechnology Co., Ltd.).
[0073] 1. Grouping: divided into blank group, control group and sample group.
[0074] 2. Experimental method.
[0075] 2.1 Sample preparation.
[0076] Precisely weigh the sample and 0.11 mg of physostigmine, respectively, and use methanol as a solvent to prepare five gradient concentrations of 2.5 μM, 5.0 μM, 10.0 μM, 20.0 μM and 40.0 μM. Accur...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com