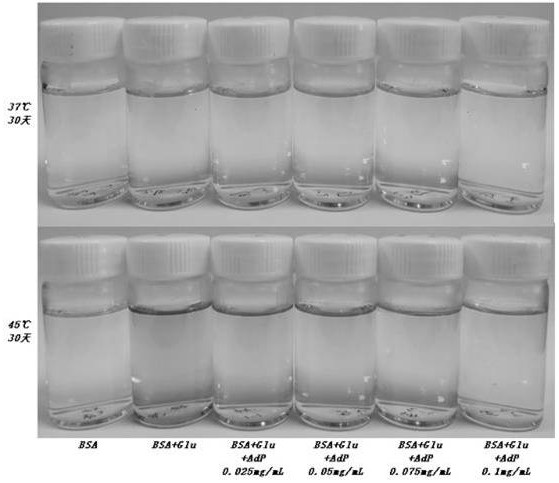
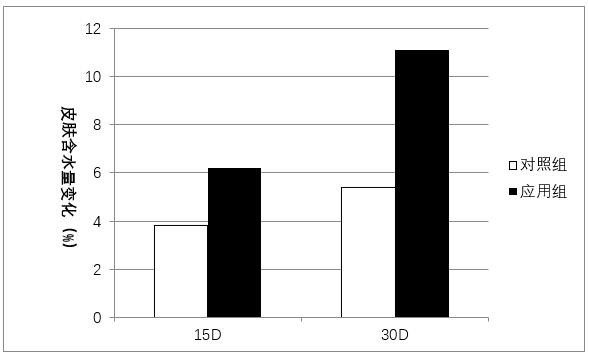
Preparation and application of antiglycation oligopeptide-5 (AdP)
An anti-glycation and oligopeptide technology is applied in the preparation methods of peptides, cosmetic preparations, preparations for skin care, etc., to achieve the effects of inhibiting protein glycosylation, low equipment cost and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The activated recombinant expression strain ER2566-pET28a-AdP was inoculated in the LB medium containing Kan resistance according to the addition amount of 3%, 27°C, 100rpm, shaker culture, when A600=1.0. Put the seed medium into the fermenter according to the inoculation amount of 10%, set the temperature at 27°C, rotate at 200rpm, adjust the pH with ammonia water, when A600=7.0, add 5mL of 0.5mmol / L IPTG for induction, and induce for 5h Afterwards, the target protein AdP is expressed in the strain.
[0034]The fermentation broth was centrifuged at 8,000 rpm for 30 min at 4°C with a refrigerated centrifuge, the supernatant was discarded, and the precipitate was stored at 4°C for future use. Take an appropriate amount of precipitate, resuspend the dispersed bacteria with 20 times the amount of Tris-Hcl buffer, process it once with a high-pressure homogenizer, and then centrifuge at 8000rpm for 30min at 2°C in a refrigerated centrifuge to collect the supernatant. Use a ...
Embodiment 2
[0036] The activated recombinant expression strain ER2566-pET28a-AdP was inoculated in the LB medium containing Kan resistance according to the addition amount of 1%, 37°C, 200rpm, shaker culture, when A600=1.0~1.5. Put the seed medium into the fermenter according to the inoculation amount of 8%, set the temperature at 37°C, rotate at 300rpm, adjust the pH with ammonia water, when A600=7.8, add 7mL of 0.5mmol / L IPTG for induction, and induce for 3h Afterwards, the target protein AdP is expressed in the strain.
[0037] The fermentation broth was centrifuged at 12,000 rpm for 25 min at 4°C with a refrigerated centrifuge, the supernatant was discarded, and the precipitate was stored at 4°C for future use. Take an appropriate amount of precipitate, resuspend and disperse the bacteria with 20 times the amount of Tris-Hcl buffer, process 3 times with a high-pressure homogenizer, and then use a refrigerated centrifuge at 12,000 rpm for 25 minutes at 4°C to collect the supernatant. ...
Embodiment 3
[0039] The activated recombinant expression strain ER2566-pET28a-AdP was inoculated in the LB medium containing Kan resistance according to the addition amount of 1%, cultured on a shaker at 42°C, 100 rpm, when A600=2.0. Put the seed medium into the fermenter according to the inoculation amount of 6%, set the temperature at 42°C, rotate at 500rpm, adjust the pH with ammonia water, when A600=9.0, add 10mL of 0.5mmol / L IPTG for induction, and induce for 2h Afterwards, the target protein AdP is expressed in the strain.
[0040] The fermentation broth was centrifuged at 15,000 rpm for 10 min at 4°C with a refrigerated centrifuge, the supernatant was discarded, and the precipitate was stored at 4°C for future use. Take an appropriate amount of precipitate, resuspend and disperse the bacteria with 20 times the amount of Tris-Hcl buffer, process 5 times with a high-pressure homogenizer, and then use a refrigerated centrifuge at 10°C at a speed of 15,000 rpm for 10 minutes to collect ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com