Design synthesis method and application of electrochemical luminophor based on covalent organic framework
A covalent organic framework, electrochemical technology, applied in chemical instruments and methods, chemiluminescence/bioluminescence, electrochemical variables of materials, etc., can solve the problem of insufficient sensitivity to accurately monitor environmental traces, time-consuming and labor-intensive processing, high requirements for instruments, etc. problems, to achieve the effects of sensitive analysis and environmental protection, ultra-low detection limit, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
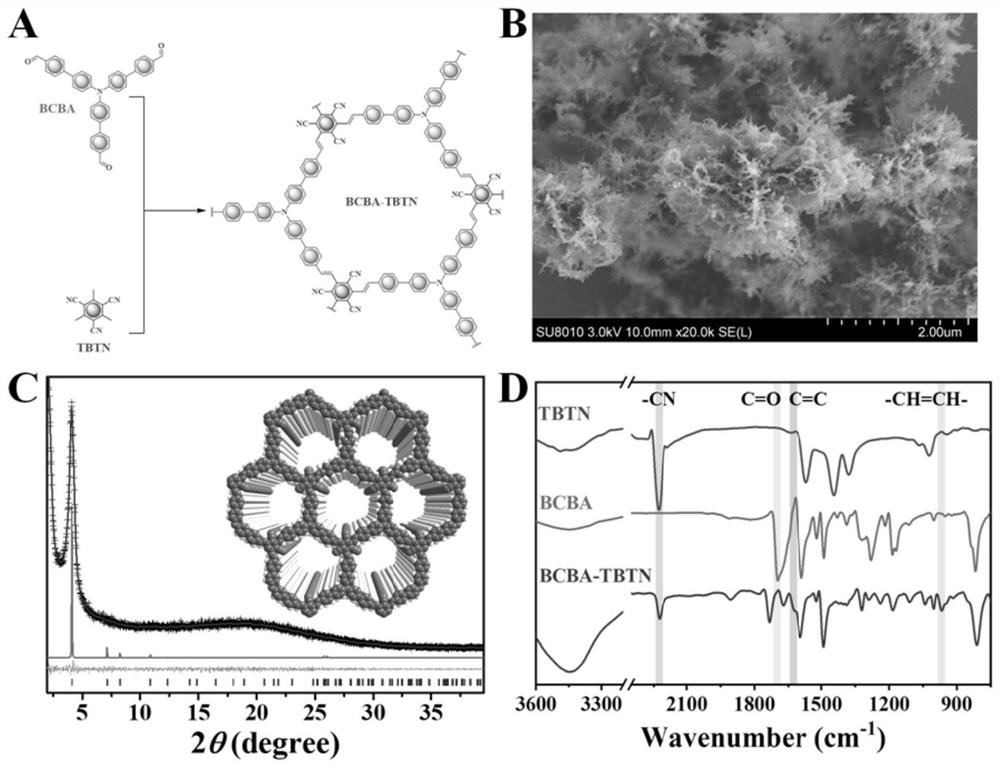
[0041] Example 1: Preparation and characterization of an olefin-linked donor-acceptor fully conjugated covalent organic framework (BCBA-TBTN)
[0042] Alkene-linked donor-acceptor fully conjugated covalent organic frameworks are prepared as figure 1 As shown in A.
[0043] Add 19.5 mg of 2,4,6-trimethylbenzene-1,3,5-tricarbonitrile (TBTN), 55.7 mg of trialdehyde teraniline (BCBA), 4.0 mL of anhydrous N into a 20 mL pyrex tube, N-dimethylformamide (DMF) and 51.1 mg piperidine, degas the mixture by three freeze-pump-thaw cycles, seal under vacuum, stir for 10 min, heat in an oil bath at 180 °C for 3 days, and wait for the reaction After the mixture was cooled to room temperature, the precipitate was collected by centrifugation, and the precipitate was washed several times with methanol, dichloromethane and tetrahydrofuran, respectively, and the washed precipitate was Soxhlet extracted in dichloromethane and tetrahydrofuran for 24 hours. Vacuum dried for 12 hours to obtain BCBA...
Embodiment 2
[0045] Embodiment 2: Construction and characterization of BCBA-TBTN / GCE system
[0046] Wipe the surface of the glassy carbon electrode (GCE) with ultrapure water-soaked filter paper, and then polish it on suede containing 1.0 μm, 0.3 μm and 0.05 μm alumina paste until the GCE surface is mirror-like, and place the electrodes in the volume HNO at a ratio of 1:1 3 :H 2 O, absolute ethanol and ultrapure water with 40% power for 1 minute, the cleaned GCE was blown dry with nitrogen; 10 μL of 1 mM BCBA-TBTN DMF solution was drop-coated on the cleaned GCE surface, Dry naturally at room temperature to make BCBA-TBTN modified GCE electrode, that is, the ECL system based on BCBA-TBTN / GCE.
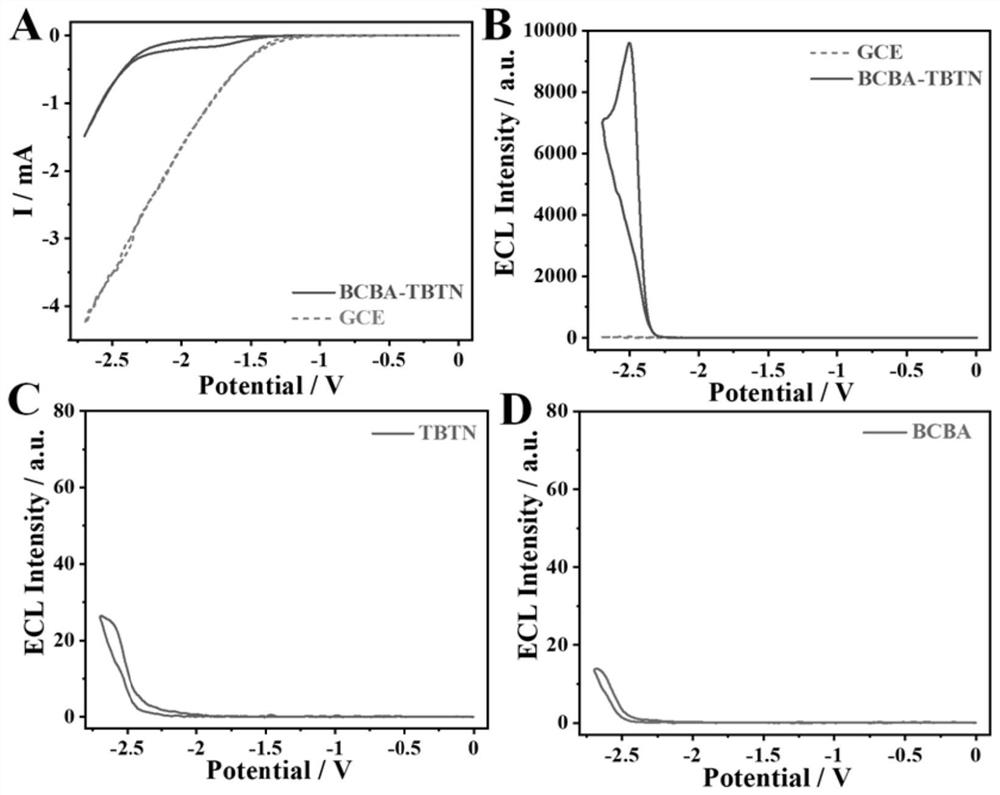
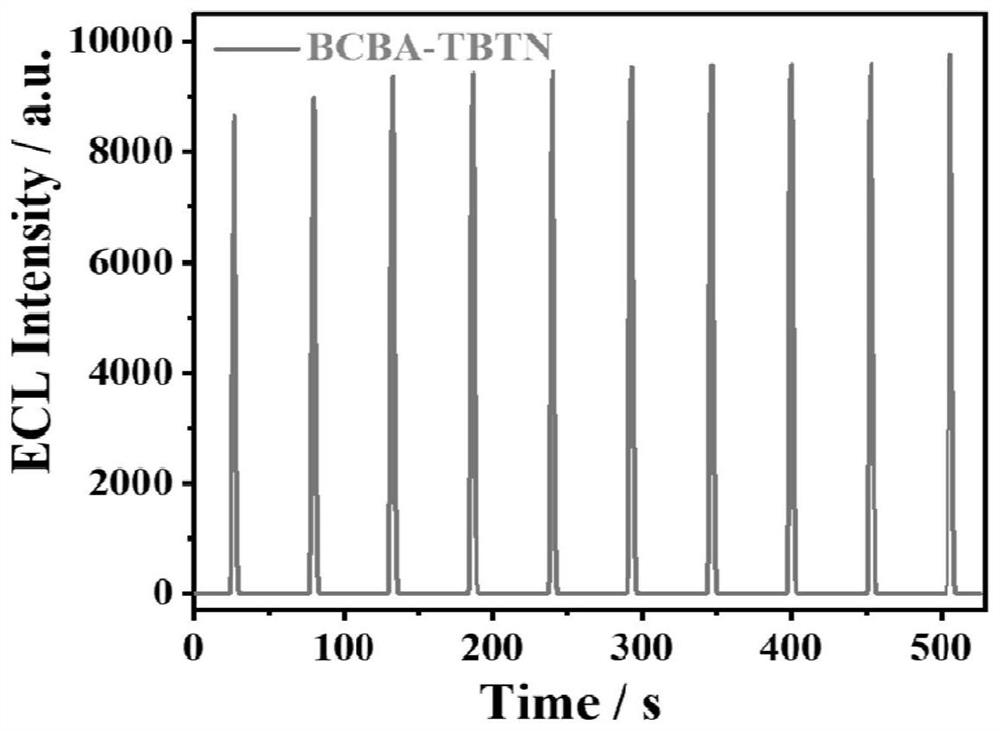
[0047] figure 2 A is the cyclic voltammetry (CV) curve of the BCBA-TBTN / GCE system constructed by the method of the present invention, the initial reduction potential of BCBA-TBTN is-1.45V, and the process of electron injection into COF to obtain free radical anion starts ECL, without adding In...
Embodiment 3
[0050] Example 3: Preparation and Characterization of Amidoxime Functionalized BCBA-TBTN (BCBA-TBTN-AO) Modified Electrode
[0051] Swell 0.4g of BCBA-TBTN in 40mL of absolute ethanol for 20min, add 1.0g of hydroxylamine hydrochloride and 1.5g of triethylamine, stir at 85°C for 1 day, filter, wash with ultrapure water, and dry under vacuum at 60°C 12 hours, get BCBA-TBTN-AO ( Figure 4 A). After weighing, re-disperse with DMF and sonicate for 2 hours to disperse evenly to obtain a DMF solution of BCBA-TBTN-AO. Take 10 μL of 1 mM BCBA-TBTN-AO solution in DMF and drop-coat it on the cleaned GCE surface, and let it dry naturally at room temperature to prepare BCBA-TBTN-AO / GCE.
[0052] Figure 4 B is the FTIR spectrum of BCBA-TBTN and BCBA-TBTN-AO. Depend on Figure 4 B Visible, BCBA-TBTN-AO at 2220cm -1 The characteristic of BCBA-TBTN-CN stretching band disappears, and at about 1260cm -1 A new stretching vibration peak of the amidoxime group appeared at , indicating the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com