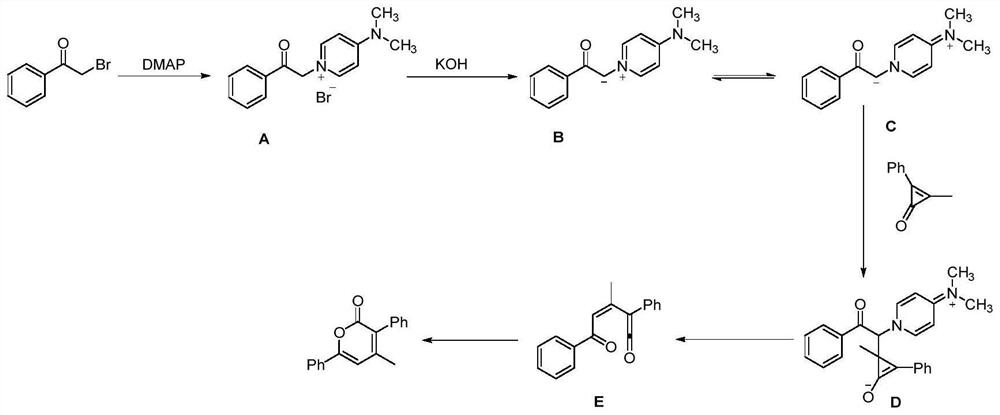
Method for synthesizing alpha-pyrone compound
A synthesis method and compound technology, applied in organic chemistry and other directions, can solve the problems of complex raw materials, lengthy steps, troublesome operation, etc., and achieve the effects of simple operation, consistent reaction conditions and high conversion rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
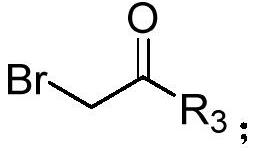
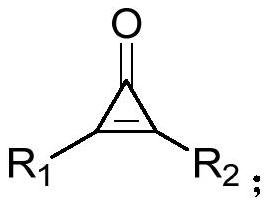
[0038] Under nitrogen, diphenylcyclopropenone (0.2mmol), 2-bromo-1-4-(trifluoromethoxy) phenylethanone (0.3mmol), DMAP (20mol%), KOH (0.3 mmol), was added to 100mg of 4A molecular sieves (the purpose was to remove water, and the reaction was controlled under anhydrous conditions) and 1,4-dioxane (2.0mL), and it was heated at 60°C for 12h, After completion of the reaction, cooling to room temperature, filtration, concentration of the reaction mixture by vacuum distillation and purification by flash column chromatography (silica gel, mixture of petroleum ether / ethyl acetate, 20:1, v / v) afforded the desired pure product 67.3 mg, the yield is 82%, and the product is a yellow solid with a melting point of 134-136°C. Its structural formula is:
[0039]
[0040] 1 H NMR (400MHz, CDCl 3 )(δ,ppm):7.93(d,J=8.9Hz,2H,Ar-H),7.38–7.13 (m,13H,Ar-H),6.82(s,1H,Ar-H).
[0041] 13 C{1H}NMR (400MHz, CDCl 3 ;δ, ppm): 162.4, 156.7, 152.5, 150.7, 137.5, 133.5, 130.8, 129.8, 128.8, 128.6, 12...
Embodiment 2
[0045] According to the method described in Example 1, the difference is that the substrates used are: diphenylcyclopropenone (0.2mmol), 2-bromo-1-4-(trifluoromethyl)phenylethanone (0.3mmol), DMAP (20mol%), KOH (0.3mmol), 100 mg of 4A molecular sieves and 1,4-dioxane (2.0mL) yielded 59.5 mg of product with a yield of 76%. The product was a yellow solid with a melting point of 170 ~172°C. Its structural formula is:
[0046]
[0047] 1 H NMR (400MHz, CDCl 3)(δ,ppm):8.03(d,J=8.2Hz,2H,Ar-H),7.74(d,J=8.3Hz,2H,Ar-H),7.32–7.15(m,10H,Ar-H ),6.93(s,1H,Ar-H).
[0048] 13 C{1H}NMR (400MHz, CDCl 3 ;δ, ppm): 162.2, 156.3, 152.2, 137.3, 134.5, 133.4, 130.7, 128.9, 128.6, 128.4, 1278.0 128.0, 126.0, 125.9, 125.7, 124.3, 106.3.
[0049] IRν max (neat):1697,1537,1329,1160,1117,1072,831,789,699cm -1 .
[0050] HRMS (ESI) calcd for C 24 h 15 f 3 o 2 [M+H]+:393.1097,found:393.1100
Embodiment 3
[0052] According to the method described in Example 1, the difference is that the substrates used are: diphenylcyclopropenone (0.2mmol), 2-bromo-1-cyclohexyl ethyl ketone (0.3mmol), DMAP (20mol%), KOH ( 0.3mmol), the heating temperature was 65°C, 100mg of 4A molecular sieve and 1,4-dioxane (2.0mL) gave 27.8mg of the product, the yield was 42%, the product was a white solid, and the melting point was 124~125°C . Its structural formula is:
[0053]
[0054] 1 H NMR (400MHz, CDCl 3 )(δ,ppm):7.25–7.07(m,10H,Ar-H),6.13(s,1H,Ar-H),2.49(t,J=11.6Hz,1H,CH),2.07(d,J =13.2Hz,2H,CH 2 ), 1.86 (d, J=12.9 Hz, 2H, CH 2 ), 1.75 (d, J=15.2Hz, 1H, CH 2 ),1.57–1.18(m,5H,CH 2 ).
[0055] 13 C{1H}NMR (400MHz, CDCl 3 ;δ,ppm): 167.89, 163.63, 152.84, 137.97, 134.10, 130.93, 128.77, 128.64, 128.32, 128.02, 127.57, 122.18, 104.50, 42.32, 30.65, 25.87.
[0056] IRν max (neat):2929,2853,1699,1634,1538,944,764,606,564cm -1 .
[0057] HRMS (ESI) calcd for C 23 h 22 o 2 [M+H]+:331.1693,f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com