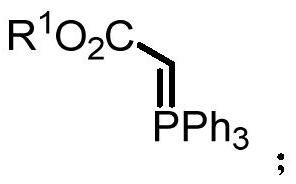
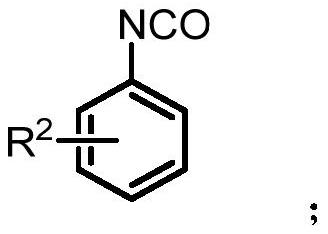
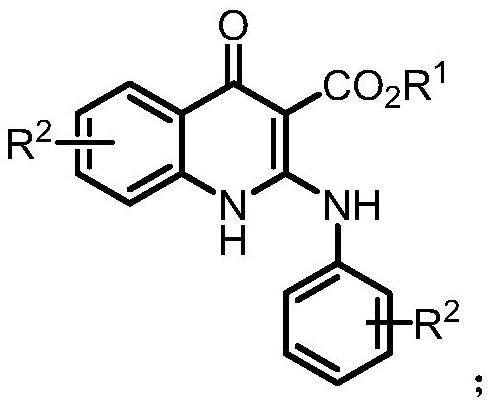
A kind of synthetic method of 2-aminoquinolone compound
An aminoquinolone and a synthesis method technology, applied in the field of synthesis of 2-aminoquinolone compounds, can solve the problems of lack of synthesis method, lengthy steps, troublesome operation and the like, and achieve the effects of consistent reaction conditions, simple operation and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Under nitrogen protection, 4-methylphenylisocyanate (0.5mmol) was added to ethoxyformylmethylenetriphenylphosphine (0.5mmol) in 1,2-dichloroethane (3mL) at 100°C Stir for 36-48 hours. After the reaction, cool to room temperature; concentrate by rotary evaporation, and perform column chromatography to obtain 124 mg of the product with a yield of 74%. The product is a yellow solid with a melting point of 222-224°C.
[0062] 1 H NMR (400MHz, DMSO-d 6 )δ10.69(br s,1H),10.60(br s,1H),7.80(s,1H),7.42(d,J=8.3Hz,1H),7.33(d,J=7.5Hz,1H), 7.28(m,4H),4.21(q,J=7.0Hz,2H),2.35(s,3H),2.34(s,3H),1.26(t,J=7.1Hz,3H).
[0063] 13 C NMR (100MHz, DMSO-d 6 )δ173.6, 169.6, 153.3, 135.2, 135.1, 134.3, 132.7, 132.0, 130.3, 124.9, 124.2, 123.9, 117.4, 93.3, 59.6, 20.6, 20.6, 14.3.
[0064] IRν max (neat):2956,1632,1578,1514,1434,1365,1281,1200,1148,1096,1018,807,742,665,556,541,520,489cm -1 .
[0065] HRMS (ESI) calcd for C 20 h 21 N 2 o 3 [M+H] + :337.1547,found:337.1540.
Embodiment 2
[0067] According to the method described in Example 1, the difference is that the substrates used are: 4-phenoxyphenyl isocyanate (0.5mmol), ethoxyformyl methylene triphenylphosphine (0.5mmol) and 1,2-bis Ethyl chloride (3mL) yielded 118mg of the product with a yield of 48%. The product was a yellow solid with a melting point of 157-159°C.
[0068] 1 H NMR (400MHz, DMSO-d 6 )δ10.82(br s,1H),10.59(br s,1H),7.58(d,J=8.9Hz,1H),7.43(ddd,J=16.3,7.1,2.7Hz,7H),7.31(dd ,J=8.9,2.8Hz,1H),7.18(t,J=7.4Hz,2H),7.11–7.03(m,6H),4.20(q,J=7.0Hz,2H),1.25(t,J= 7.1Hz, 4H).
[0069] 13 C NMR (100MHz, DMSO-d 6 )δ 172.9, 169.4, 156.6, 156.6, 154.7, 153.4, 152.7, 133.3, 132.2, 130.1, 130.1, 126.5, 125.2, 123.7, 123.6, 123.5, 119.8, 119.6, 118.8, 118.9, 93.7, 112
[0070] IRν max (neat):2977,1632,1582,1524,1503,1485,1368,1303,1280,1217,1161,1095,1051,872,844,807,768,742,706,688,566,523,506,428cm -1 .
[0071] HRMS (ESI) calcd for C 30 h 25 N 2 o 5 [M+H] + :493.1758,found:493.1762.
Embodiment 3
[0073] According to the method described in Example 1, the difference is that the substrates used are: 4-trifluoromethylphenylisocyanate (0.5mmol), ethoxyformylmethylenetriphenylphosphine (0.5mmol) and 1,2- Dichloroethane (3 mL) yielded 144 mg of the product with a yield of 65%. The product was a yellow solid with a melting point of 124-126°C.
[0074] 1 H NMR (400MHz, CDCl 3 )δ12.79(br s,1H),9.54(br s,1H),8.36(s,1H),7.92(d,J=8.5Hz,2H),7.76(dd,J=8.8,2.1Hz,1H ), 7.62(dd, J=18.9, 8.7Hz, 3H), 4.59(q, J=7.1Hz, 2H), 1.58(t, J=7.1Hz, 3H).
[0075] 13 C NMR (100MHz, CDCl 3 )δ169.8, 169.3, 152.6, 142.6, 129.0 (q, J = 10Hz), 128.2127.1, 126.1 (q, J = 12Hz), 124.2 (q, J = 289Hz), 125.1, 124.8, 122.9, 122.8, 121.9 (q, J=13Hz), 116.9, 93.6, 63.5, 14.1.
[0076] IRν max (neat):3392,1660,1635,1597,1547,1443,1415,1316,1257,1150,1110,1064,1011,950,908,870,850,834,730,609,525,414cm -1 .
[0077] HRMS (ESI) calcd for C 20 h 15 f 6 N 2 o 3 [M+H] + :445.0982,found:445.0974.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com