Rifampicin freeze-dried powder needle for injection and production method thereof
A technology for freeze-dried powder injection and production method, applied in the field of medicine, can solve the problems of uncontrolled alkali addition speed, high overall energy consumption, potential safety hazards, etc., and achieve the effects of being beneficial to stability, high freeze-drying efficiency, and ensuring safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
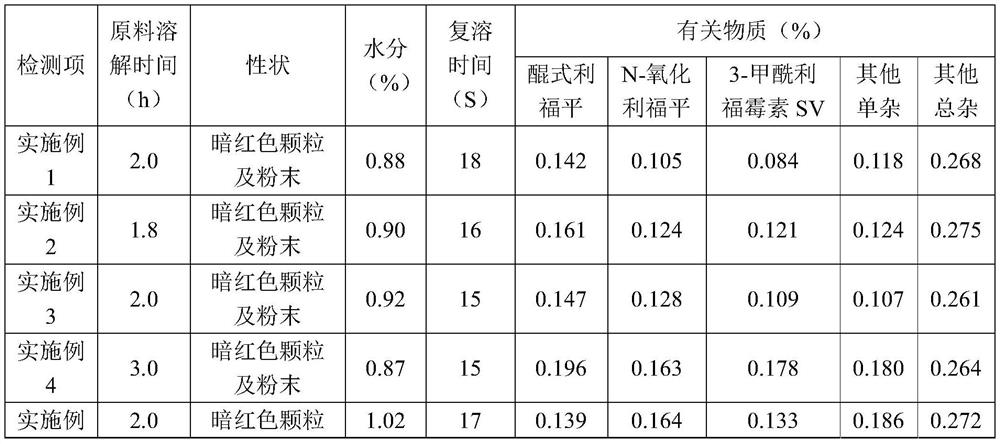
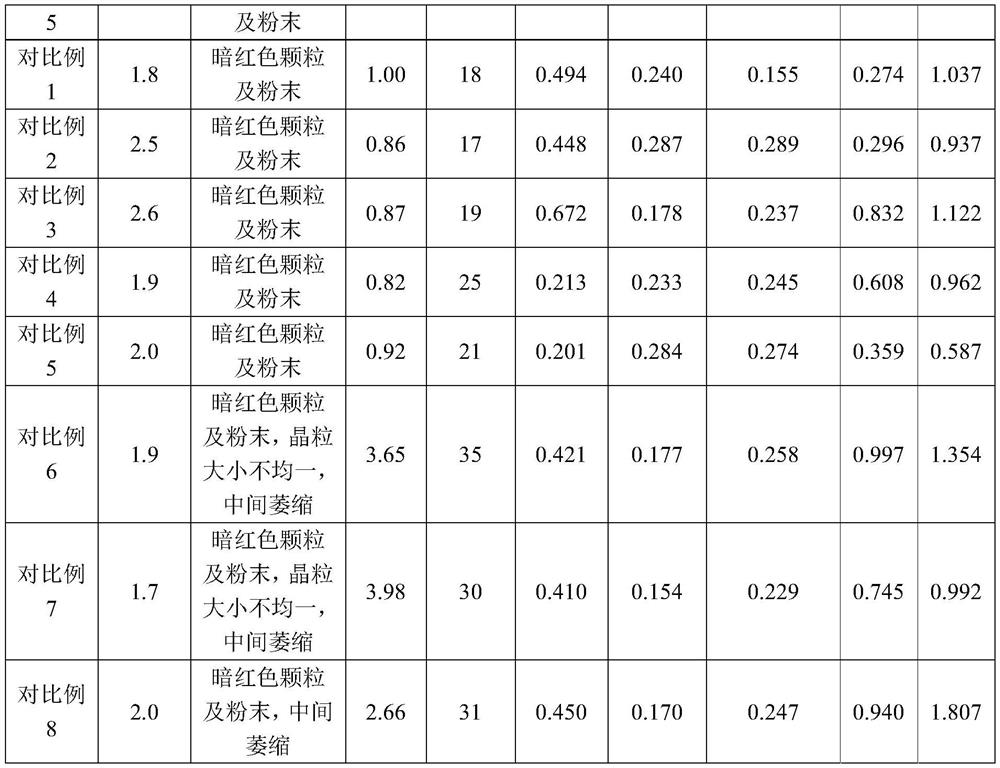
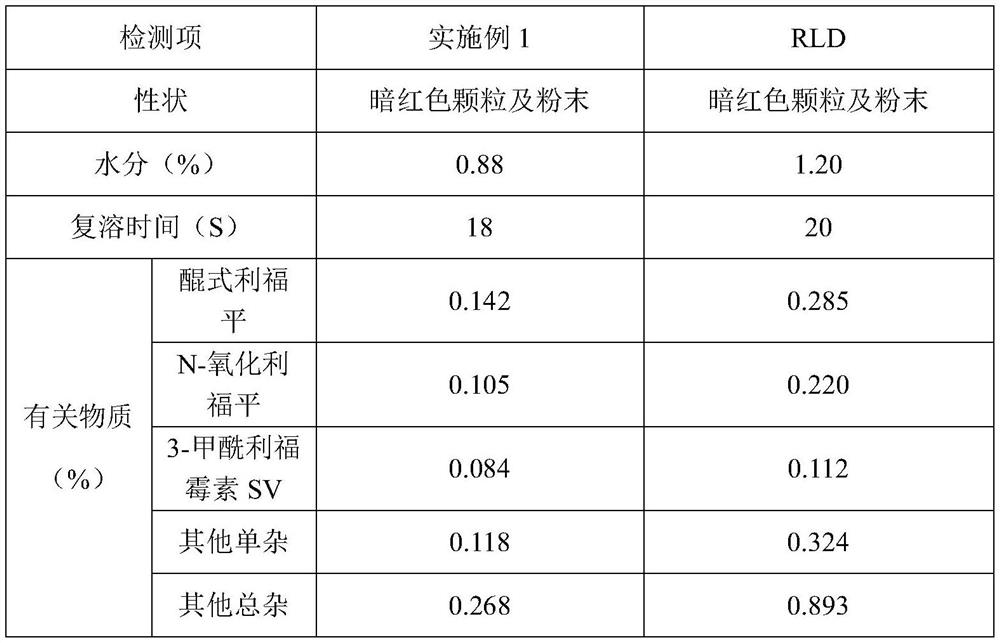
[0050] A rifampicin freeze-dried powder for injection, made of sodium formaldehyde sulfoxylate, rifampicin, sodium hydroxide and water for injection at a temperature of 10°C, sterilizing and filtering, filling and freeze-drying After that, the finished product is obtained, wherein, in the preparation process of the medicinal liquid, its pH value is adjusted to 8.4 with a pH regulator, and every 1000 parts of the medicinal liquid contains the following components by weight: the active raw material rifampicin 120 parts, 2 parts of sodium formaldehyde sulfoxylate, 4.3 parts of sodium hydroxide.
[0051] The detailed preparation process is as follows:
[0052] (1) Add a total of 80% of water for injection at 10°C in the liquid preparation tank, start stirring, and under the condition of maintaining the temperature at 10°C, add 2 parts of sodium formaldehyde sulfoxylate per 1000 parts of the liquid medicine, dissolve After complete, slowly add 120 parts of rifampicin, stir to disp...
Embodiment 2
[0065] A rifampicin freeze-dried powder for injection, made of sodium formaldehyde sulfoxylate, rifampicin, sodium hydroxide and water for injection at a temperature of 20°C, sterilizing and filtering, filling and freeze-drying After that, the finished product is obtained, wherein, in the preparation process of the medicinal liquid, its pH value is adjusted to 8.4 with a pH regulator, and every 1000 parts of the medicinal liquid contains the following components by weight: the active raw material rifampicin 120 parts, 2 parts of sodium formaldehyde sulfoxylate, 4.3 parts of sodium hydroxide.
[0066] The detailed preparation process is as follows:
[0067] (1) Add a total of 80% of water for injection at 20°C in the liquid preparation tank, start stirring, and under the condition of maintaining the temperature at 20°C, add 2 parts of sodium formaldehyde sulfoxylate per 1000 parts of the liquid medicine, dissolve After complete, slowly add 120 parts of rifampicin, stir to disp...
Embodiment 3
[0080] A rifampicin freeze-dried powder for injection, made of sodium formaldehyde sulfoxylate, rifampicin, sodium hydroxide and water for injection at a temperature of 10°C, sterilizing and filtering, filling and freeze-drying After that, the finished product is obtained, wherein, in the preparation process of the medicinal liquid, its pH value is adjusted to 8.6 with a pH regulator, and every 1000 parts of the medicinal liquid contains the following components by weight: the active raw material rifampicin 120 parts, 2 parts of sodium formaldehyde sulfoxylate, 5.0 parts of sodium hydroxide.
[0081] The detailed preparation process is as follows:
[0082] (1) Add a total of 80% of water for injection at 10°C in the liquid preparation tank, start stirring, and under the condition of maintaining the temperature at 10°C, add 2 parts of sodium formaldehyde sulfoxylate per 1000 parts of the liquid medicine, dissolve After complete, slowly add 120 parts of rifampicin, stir to disp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com