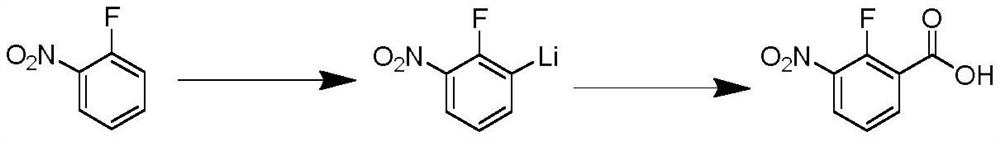
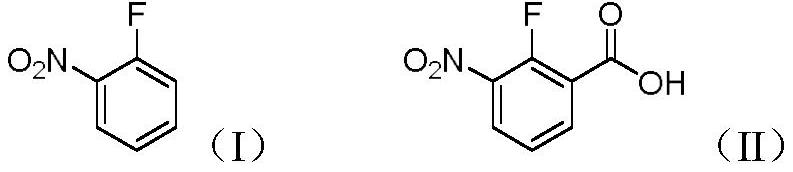
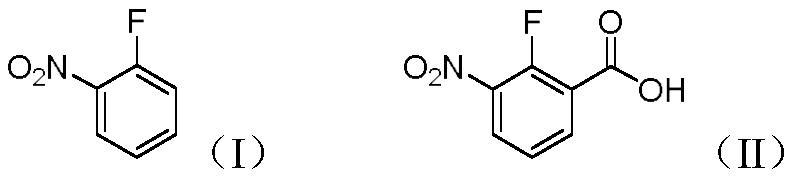Preparation method of 2-fluoro-3-nitrobenzoic acid
A technology of nitrobenzoic acid and fluoronitrobenzene, which is applied in the field of preparation of 2-fluoro-3-nitrobenzoic acid, can solve the problems of poor selectivity and low yield, achieve mild reaction conditions, high yield, Effects suitable for large-scale preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 250ml three-necked flask, dissolve 2-fluoronitrobenzene (1.41g, 0.01mol) in 2-methyltetrahydrofuran (100ml), replace it with a nitrogen atmosphere, and start to lower the temperature. When the temperature is -30°C, start A mixed solution of lithium diisopropylamide (2.14g, 0.02mol) and 2-methyltetrahydrofuran (40ml) was added dropwise. After the dropwise addition, the reaction was continued at this temperature for 6h. After the reaction was completed, under a nitrogen atmosphere, Dry ice (2.2 g, 0.05 mol) was added quickly to quench and stirring was continued for 2 h. Then the reaction liquid was naturally returned to room temperature, and the solvent was removed in vacuo, and 30 ml of water was added to the obtained residue, and the pH of the aqueous phase was adjusted to 2 with concentrated hydrochloric acid, and then extracted with ethyl acetate (30 ml×3). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtere...
Embodiment 2
[0027] In a 250ml three-necked flask, dissolve 2-fluoronitrobenzene (1.41g, 0.01mol) in tetrahydrofuran (100ml), replace it with a nitrogen atmosphere, and start to lower the temperature. When the temperature is at -80°C, start to add diiso A mixed solution of lithium propylamide (8.57g, 0.08mol) and tetrahydrofuran (40ml) was added dropwise, and the reaction was continued at this temperature for 8h. After the reaction was completed, dry ice (2.2g, 0.05 mol), quenched and continued to stir for 2h. Then the reaction liquid was naturally returned to room temperature, and the solvent was removed in vacuo, and 30 ml of water was added to the obtained residue, and the pH of the aqueous phase was adjusted to 2 with concentrated hydrochloric acid, and then extracted with ethyl acetate (30 ml×3). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain 1.64 g of 2-fluoro-3-nitrobenzoic acid wi...
Embodiment 3
[0029] In a 250ml three-necked flask, dissolve 2-fluoronitrobenzene (1.41g, 0.01mol) in ether (100ml), replace it with a nitrogen atmosphere, and then start to lower the temperature. When the temperature is at -80°C, start to drop tert-butyl Lithium (3.84g, 0.06mol) and ether (40ml) mixed solution, after the dropwise addition, continue to react at this temperature for 2h, after the reaction, under nitrogen atmosphere, quickly add dry ice (2.2g, 0.05mol) , was quenched and continued to stir for 2h. Then the reaction liquid was naturally returned to room temperature, and the solvent was removed in vacuo, and 30 ml of water was added to the obtained residue, and the pH of the aqueous phase was adjusted to 2 with concentrated hydrochloric acid, and then extracted with ethyl acetate (30 ml×3). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered with suction, and concentrated to obtain 0.96 g of 2-fluoro-3-nitrobenzoic acid wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com