Difunctional lithium metal battery electrolyte and application thereof
A lithium metal battery and electrolyte technology, applied in lithium batteries, electrolytes, secondary batteries, etc., can solve the problem of not being able to meet the stability and high-voltage performance of lithium negative electrodes at the same time, and achieve improved conductivity and electrode wettability. High commercial Value, the effect of strong bonding ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
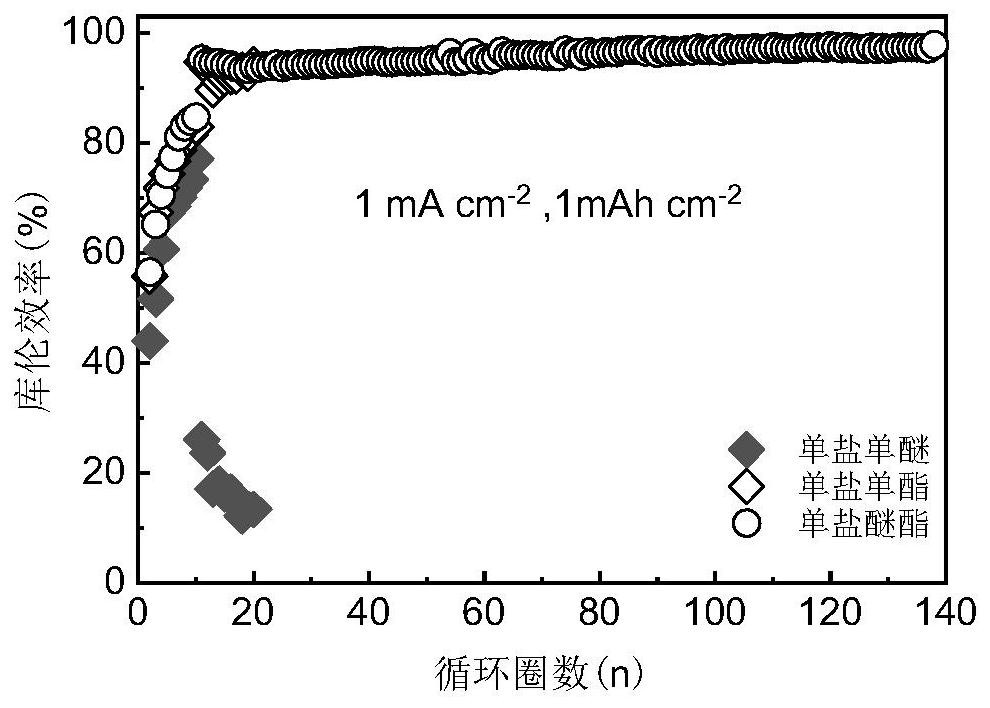
[0042] Monoether electrolyte (1.5M LiBF 4 in DME, v / v=1), monoester electrolyte (1.5MLiBF 4 in FEC, v / v=1) and bifunctional ether ester mixed electrolyte (1M LiBF 4 in FEC / DME, v / v=1), using the above electrolyte, lithium sheets, copper sheets, and separators to assemble a lithium-copper half-cell, and at 1mA cm -2 The Coulombic efficiency test is carried out under the current density, such as figure 1 As shown, in the monoester electrolyte control group, the lithium negative electrode can only cycle stably for 20 weeks, while in the monoether electrolyte control group, the lithium negative electrode cannot cycle normally after several cycles of charging and discharging. In contrast, in the bifunctional electrolyte experimental group, the Li anode can be cycled stably for more than 100 cycles with high average Coulombic efficiency. This example shows that the bifunctional electrolyte proposed by the present invention has excellent performance when used in lithium metal batt...
Embodiment 2
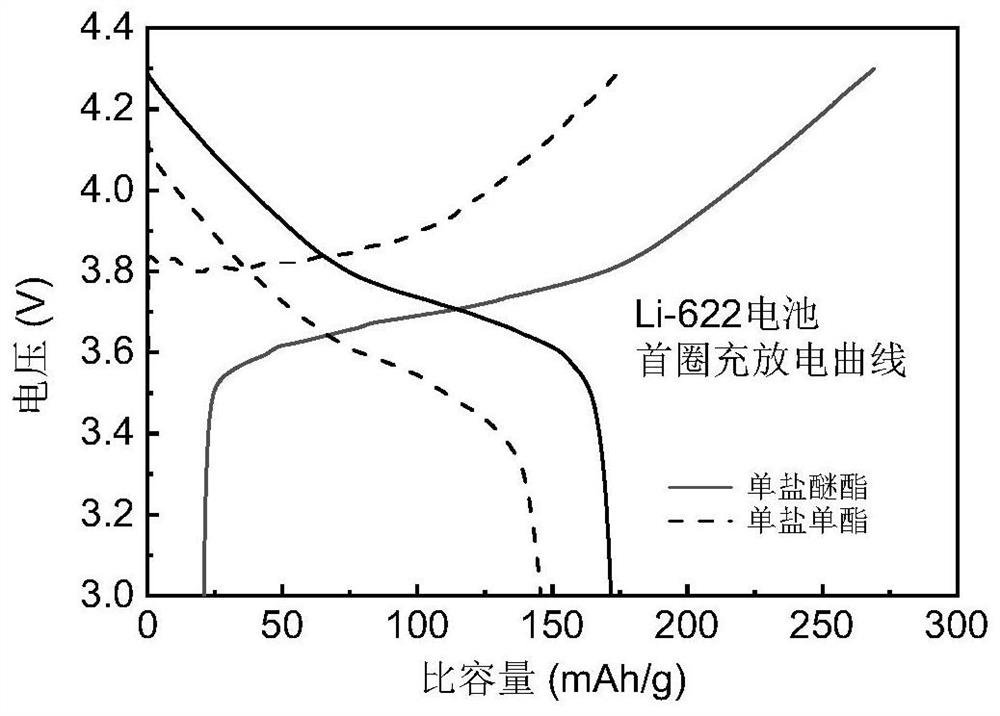
[0044] Monoether electrolyte (1.5M LiBF 4 in DME, v / v=1), monoester electrolyte (1.5MLiBF 4 in FEC, v / v=1) and bifunctional electrolyte (1M LiBF 4 in FEC / DME, v / v=1), use the above electrolyte, lithium sheet, 622 positive electrode sheet, and separator to assemble a Li-622 battery, and perform charge and discharge tests on it. The charge and discharge curve of the first cycle is as follows: figure 2 As shown, the Li-622 battery cannot be charged and discharged normally with the monoether electrolyte control group, but the Li-622 battery can be cycled normally with the monoester electrolyte and the bifunctional electrolyte, and the bifunctional electrolyte experimental group’s pole The oxidation is smaller than that of monoester electrolyte. This example shows that the performance of the bifunctional electrolyte proposed by the present invention is better than that of other monoether monoester electrolytes, and the cycle of lithium metal batteries is more stable.
Embodiment 3
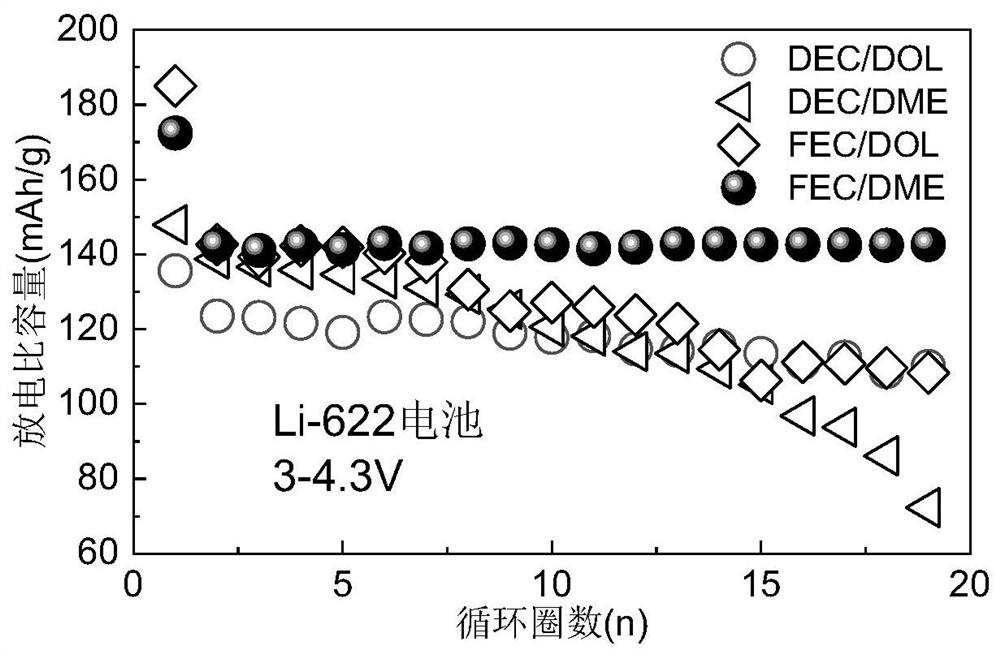
[0046] Separately configure four kinds of dual-functional electrolytes composed of different solvents, each of which is 1.5M LiBF 4 in DEC / DOL(v / v=1), 1.5M LiBF 4 in DEC / DME(v / v=1), 1.5M LiBF 4 in FEC / DOL(v / v=1), 1.5M LiBF 4 inFEC / DME (v / v=1), to explore the effect of different ether-ester mixed solvent compositions on battery performance. Use the above electrolyte, lithium sheet, 622 positive electrode sheet, and diaphragm to assemble a Li-622 battery, and conduct charge and discharge tests on it. Cyclic curves such as image 3 As shown, the cycle stability of the Li-622 battery assembled by the bifunctional electrolyte experimental group using FEC / DME (v / v=1) is the best. This example shows that the use of FEC and DME as solvents in the bifunctional electrolyte proposed by the present invention has more excellent performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com