Nucleic acid construct for gene therapy of glycometabolism-related diseases
A technology for nucleic acid constructs and sugar metabolism, applied in metabolic diseases, genetic engineering, plant genetic improvement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1: the structural design of the expression framework of the nucleic acid construct expressing GLP1 receptor agonist
[0072] Such as figure 2 As shown, the GLP1 receptor agonist carries a signal peptide sequence, figure 2 The overall sequence shown is translated in the cell as an intact precursor molecule and secreted out of the cell. The precursor molecule includes GLP1, a connecting peptide composed of three flexible unit amino acids in series, and an auxiliary peptide; wherein the auxiliary peptide can be GLP1 or GLP1-connecting peptide-GLP1, or human antibodies IgG1CH2 and IgG1CH3 or other structures. The structure of the GLP1 receptor agonist molecule used in the present invention is:
[0073] 1. Monomer gene expression framework (GLP-1), consisting of N-terminal signal peptide MALLTNLLPLCCLALLALPAQS (SEQ ID NO: 30) and a single GLP-1 (7-37) gene HAEGTFTSDVSSYLE GQAAKEFIAWLVKGRG (SEQ ID NO: 31). The constructed GLP1 receptor agonist expression frame...
Embodiment 2
[0077] Embodiment 2: Construction of the nucleic acid construct expressing GLP1 receptor agonist
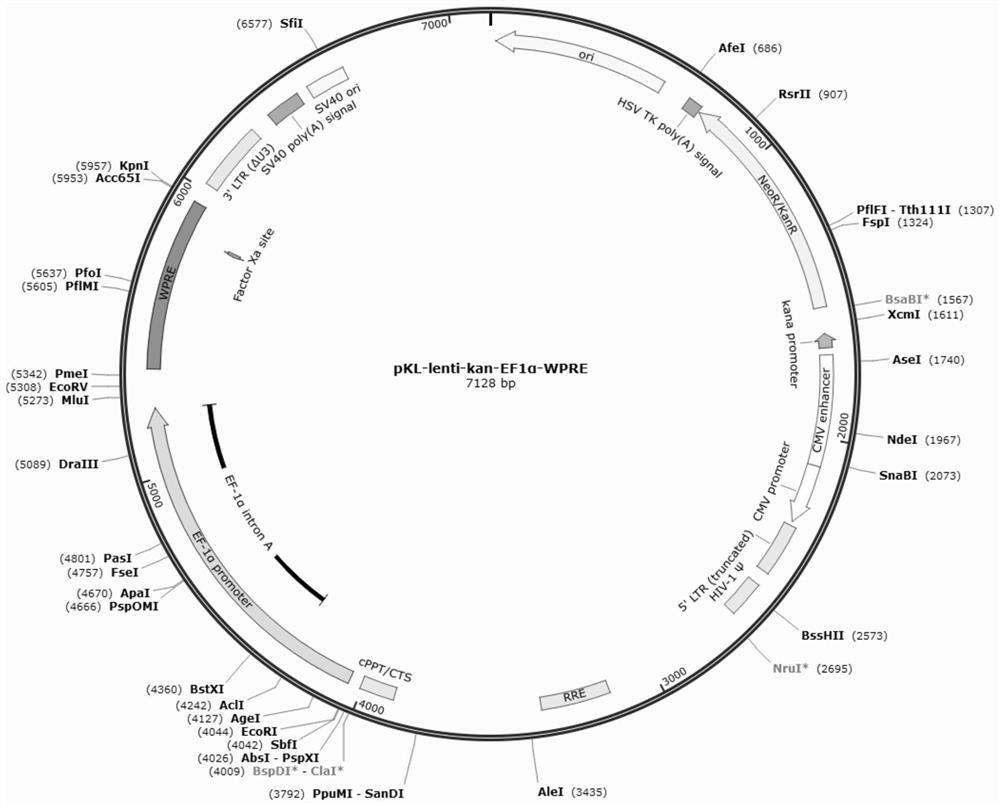
[0078] Such as Figure 3A As shown, the above-mentioned GLP1 receptor agonist gene expression framework was cloned into the latest generation of lentiviral vector backbone pKL-kan-lenti-EF1α-WPRE ( Figure 1A , the nucleotide sequence is as in SEQ ID NO:9). The lentiviral vector backbone includes: 5'LTR, wherein the promoter region of LTR is replaced with CMV promoter; ψ packaging signal; retroviral export element RRE; cPPT; promoter CBH; Polynucleotides; post-transcriptional regulatory elements are wPRE; PPT; ΔU3 3'LTR; and poly A signal. The gene expression frameworks GLP1, GLP1-Fc, GLP1-GLP1 and GLP1-GLP1-GLP1 designed in Example 1 were synthesized by General Biosystems (Anhui) Co., Ltd., and then cloned into lentivirus by homologous recombination methods well known in the art Between the multiple cloning sites EcoRI / EcoRV on the vector backbone pKL-kan-lenti-EF1α-WPRE, the ...
Embodiment 3
[0080] Example 3: Packaging and purification of viruses expressing GLP1 receptor agonists
[0081] In 293T cell line with lentiviral vectors (pKL-Kan-lenti-EF1α-GLP1, pKL-Kan-lenti-EF1α-KLDi01, pKL-Kan-lenti-EF1α-KLDi02 and pKL-Kan-lenti-EF1α-KLDi03) Packaging of lentiviral vectors for antibody gene therapy. The antibody gene lentiviral vectors constructed in Implementation 2 (pKL-Kan-lenti-CBH-GLP1, pKL-Kan-lenti-EF1α-KLDi01, pKL-Kan-lenti-EF1α-KLDi02 and pKL-Kan-lenti-EF1α- KLDi03), envelope plasmid (pKL-Kan-Vsvg, its nucleotide sequence is shown in SEQ ID NO:19) and packaging plasmid (pKL-Kan-Rev, its nucleotide sequence is shown in SEQ ID NO:20 ; pKL-Kan-GagPol, whose nucleotide sequence is shown in SEQ ID NO: 21) mixed and simultaneously co-transfected 293T cells (purchased from the American Type Culture Collection Center (ATCC), ATCC preservation number is CRL-3216), Packaging of HIV neutralizing antibody gene therapy lentivirus was carried out in this 293T cell line. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com