Molten salt electrochemical method for coproducing metal/carbon composite material and hydrogen
A carbon composite material, electrochemical technology, applied in electrodes, electrolysis components, electrolysis process and other directions, can solve the problems of incomplete reaction, high reaction temperature, difficult to control, etc., to achieve increased added value, mild reaction conditions and simple steps. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
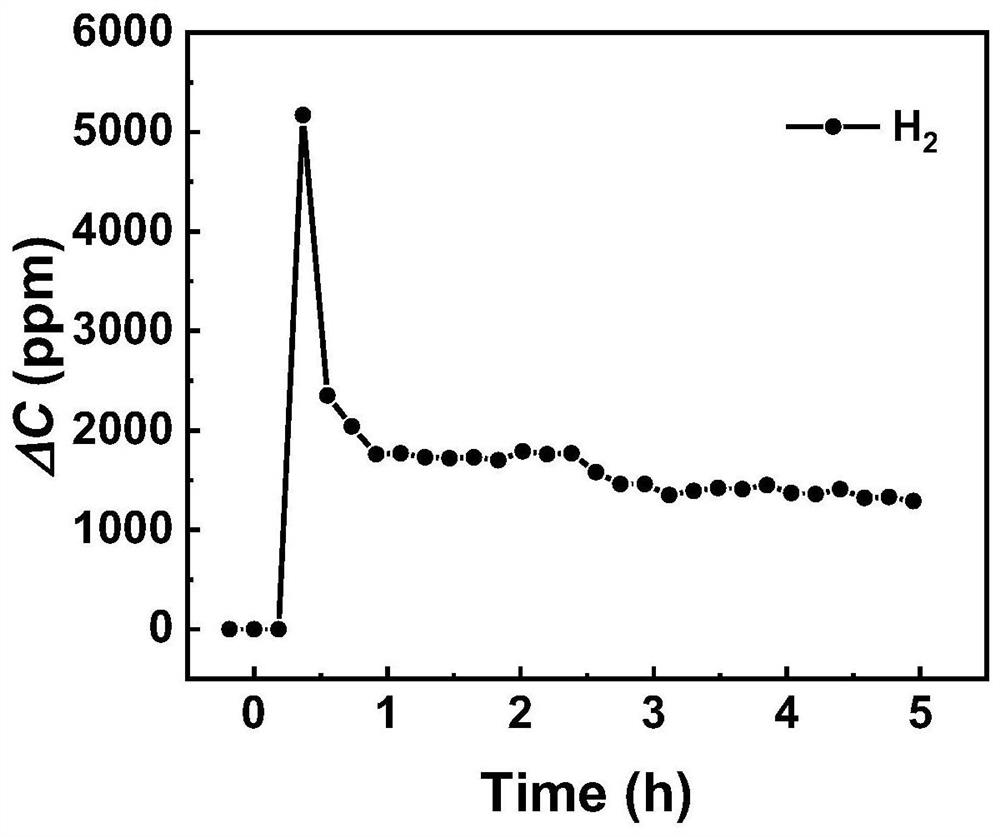
[0029] with CaCl 2 , NaCl, (molar ratio is 52:48) 500g molten salt as electrolyte, add 20g CaO, mix well and pour into crucible. First raise the temperature at 5°C / min to 250°C and keep it warm for 24 hours to completely evaporate the water in the molten salt. Then the temperature was raised to 850°C at 5°C / min, and at the same time, argon gas was introduced as a protective atmosphere, and the temperature was kept for 3 hours, and the temperature was lowered and kept at 700°C. Use the metal Au sheet as the anode, pass 3vol% methane on the surface of the metal Au sheet, use the liquid Zn as the cathode, and electrolyze with the potential of 0.8V vs. Ag / AgCl for 5 hours. (see attached image 3 ), and no other gas was detected except methane and argon, that is, methane was decomposed into hydrogen at the anode. After the electrolysis, the liquid Zn electrode was taken out and washed three times with deionized water and absolute ethanol respectively to obtain the metal Zn / carbo...
Embodiment 2
[0031] with CaCl 2 , NaCl (molar ratio is 52:48) 500g molten salt is used as the electrolyte, mix well and pour into the crucible. First raise the temperature at 5°C / min to 300°C and keep it warm for 24 hours to completely evaporate the water in the molten salt. Then the temperature was raised to 850°C at 5°C / min, and at the same time, argon gas was introduced as a protective atmosphere, and the temperature was kept for 3 hours, and then the temperature was lowered to 850°C and kept at 850°C. will CO 2 As an added oxide, it is passed into the molten salt medium at a speed of 100mL / min. The metal Ru-GDC sheet is used as the anode, and 10vol% methane is passed on the surface of the metal Ru-GDC sheet, and the liquid SnBi alloy is used as the cathode. Potential electrolysis of vs.Ag / AgCl for 1 hour, and at the same time using online gas chromatography to simultaneously monitor the synchronous precipitation of hydrogen in the anode area, removing methane and CO 2 No other gases...
Embodiment 3
[0035] With LiCl, NaCl (1:1 molar ratio) 500g molten salt as electrolyte, add 20g Na 2 O, mixed well and poured into the crucible. First raise the temperature at 5°C / min to 300°C and keep it warm for 24 hours to completely evaporate the water in the molten salt. Then the temperature was raised to 850°C at 5°C / min, and at the same time, argon gas was introduced as a protective atmosphere, and the temperature was kept for 3 hours, and then the temperature was lowered to 650°C and kept at 650°C. The metal Cu mesh is used as the anode, methane is passed on the surface of the metal Cu mesh, and the liquid Ga is used as the cathode, at 40mA / cm 2 The electric current was electrolyzed for 12 hours, and the anode potential was monitored to be 0.81vs.Ag / AgCl during the electrolysis process. Simultaneously, the anode was monitored by online gas chromatography to produce hydrogen gas synchronously, and no other gases were detected except methane. After the electrolysis, the liquid Ga e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com