Metal iridium (III) complex as well as preparation method and application thereof
A technology of metal iridium and complexes, applied in the field of biomedicine, can solve the problems of anti-tumor activity to be improved, and achieve the effects of low in vitro cell dark toxicity, high phototoxicity, and excellent photodynamic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The synthesis of embodiment 1 medicine
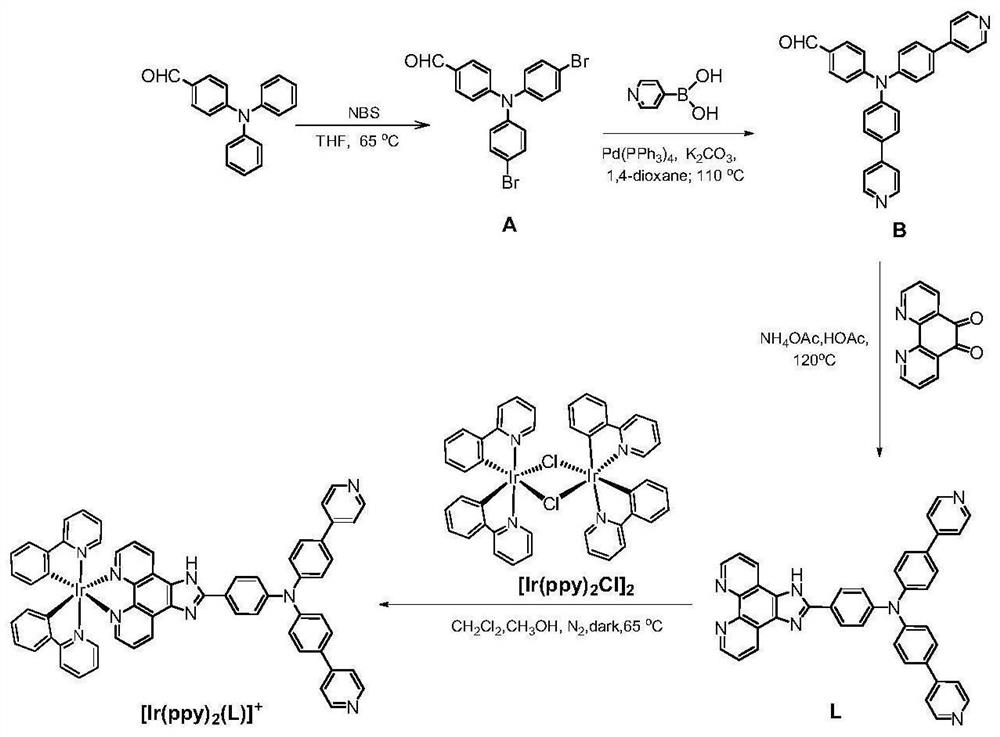
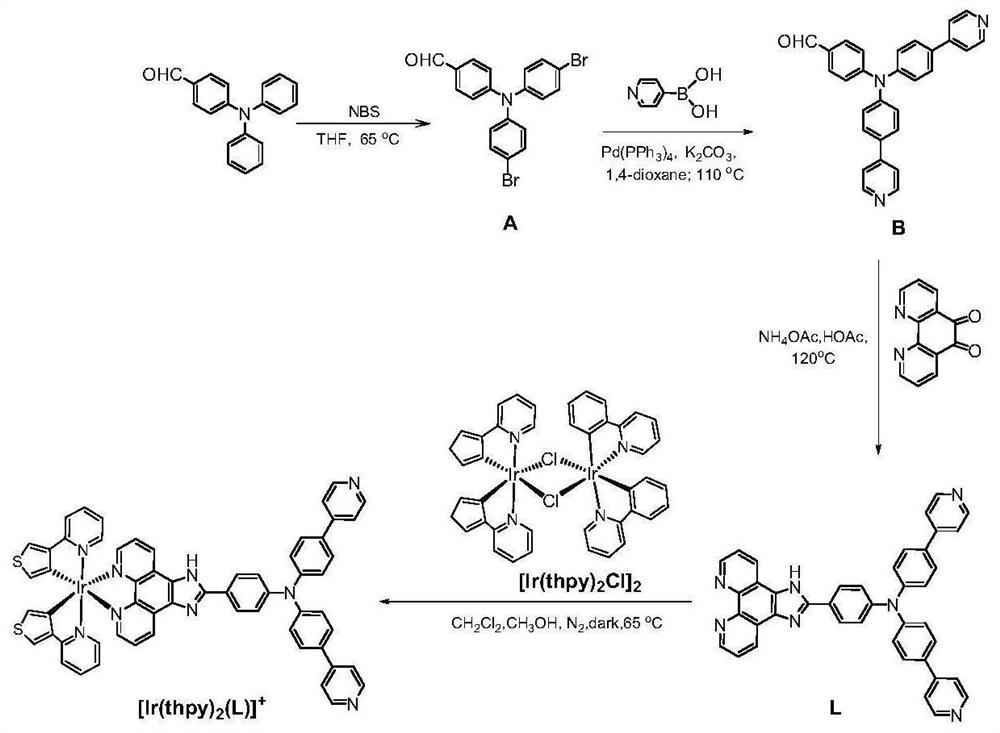
[0065] (1) Synthesis of precursors:
[0066] Cyclometallic iridium chloride bridge precursor [Ir(ppy) 2 Cl] 2 and [Ir(thpy) 2 Cl] 2 It was synthesized by literature method [Journal of the American Chemical Society, 2011, 133(29): 11231-9.].
[0067] (2) Ligand synthesis:
[0068] Take 10.93g (0.04mol) of 4-diphenylaminobenzaldehyde and dissolve it in 100mL of tetrahydrofuran, then add 18.5g (0.104mol) of N-bromosuccinimide, stir at room temperature for 24 hours, then terminate the reaction, spin the solvent under reduced pressure Column chromatography (DCM / PE=1:1, v / v) gave 9.1 g of bright yellow brominated product with a yield of 53%.
[0069] Take brominated product 1.8g (0.004mol) and 4-pyridine boronic acid 1.54g (0.0125mol), add 85mg (0.074mmol) tetrakis (triphenylphosphine) palladium catalyst, then add 12mL dissolved in 1.72g (0.012mol) carbonic acid Potassium deionized water and 40 mL of 1,4-dioxane were stirred in ...
Embodiment 2
[0083] The photophysical property of embodiment 2 complexes
[0084] Lipid-water partition coefficient determination experiment:
[0085] The mixed solution of n-octanol and water was mixed on a shaker for 24 hours, and after standing for stratification, an equal-volume biphasic solution was added to the complex, and mixed on a shaker for 48 hours. Standing and stratifying, the two-phase solution of the complex was obtained, and the precipitation was removed. Dilute the two phases with equal concentrations of methanol to measure the UV-visible absorption value respectively, and calculate lgP by formula 3 o / w value:
[0086] lgP o / w =lg(A o / A w ) Formula 3
[0087] Ao and Aw are the absorbance of the complex in n-octanol and water phase at specific wavelengths, respectively.
[0088] Phosphorescence lifetime measurement experiment:
[0089] The phosphorescence lifetime was detected with a combined fluorescence lifetime and steady-state fluorescence spectrometer produce...
Embodiment 3
[0101] The cellular uptake time dependence test of embodiment 3 complexes
[0102] Studying the uptake rate of the complex by cells can provide a reference for determining the incubation time of the complex in the next series of cell experiments.
[0103] Cell uptake time-dependent experimental steps: HeLa cells were seeded in a 35mm confocal dish and cultured for 24 hours, added 20μM complexes and incubated for different times (1h, 2h, 4h and 6h), washed twice with PBS, and immediately confocal with laser Focusing microscope observation, excitation at 405nm, collection of emission wavelengths: 620±30nm (Ir-ppy); 640±30nm (Ir-thpy).
[0104] Time-dependent confocal microscopy imaging results by cellular uptake, such as Figure 13 and Figure 14 It can be seen that the phosphorescence intensity in HeLa cells is very weak after Ir-ppy or Ir-thpy treatment for 1 h. After 2 hours of Ir-ppy treatment, obvious phosphorescence in the cells can be observed, but the intracellular ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com