Polypyridine ruthenium complex and preparing method and application thereof
A complex, ruthenium pyridine technology, applied in ruthenium organic compounds, pharmaceutical formulations, platinum group organic compounds, etc., can solve the problems of high dark toxicity and low phototoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of polypyridine ruthenium complex, comprises the steps:
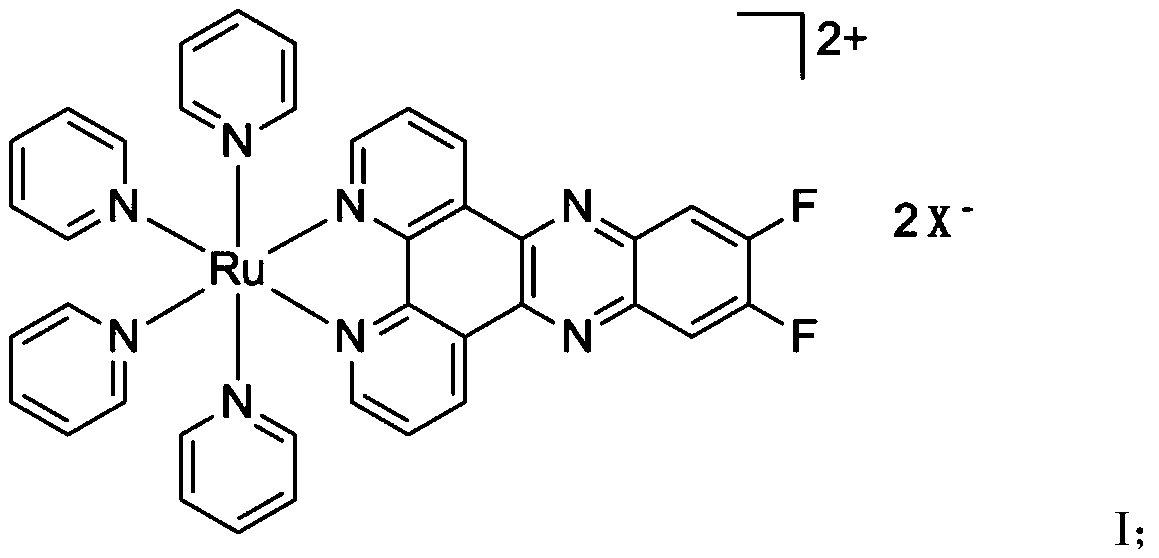
[0035] Reflux 210mg (1mmol) of 4,5-difluoro-1,2-phenylenediamine with 126mg (1mmol) of 1,10-o-phenanthrene-5,6-dione in ethanol for three hours and dissolve in ethanol 11,12-difluorobipyrido[3,2-a:2',3'-c]phenazine was obtained by recrystallization. Then 90mg (0.3mmol) of 11,12-difluorobipyrido[3,2-a:2',3'-c]phenazine was dimerized with 75mg (0.15mmol) of dichlorophenylruthenium(II) The solid was stirred overnight in 40 mL of methanol until a clear red liquid was formed, then the solvent was removed and redissolved in 40 mL of water. Add 1 mL of excess pyridine, degas with nitrogen for 30 minutes, heat to reflux for 2 hours and turn on nitrogen to cool. The product was eluted and purified on a silica gel chromatography column with acetonitrile: saturated aqueous potassium chloride solution = 10:1, and the excess potassium chloride in the column product was removed by utilizing the low sol...
Embodiment 2
[0039] Repeat Example 1, the difference is that after the purification is completed, add NH 4 PF 6 or NaClO 4 , the monovalent anion obtained by ion exchange is (PF 6 ) - or (ClO 4 ) - A polypyridine ruthenium complex having the structure described in formula I.
Embodiment 3
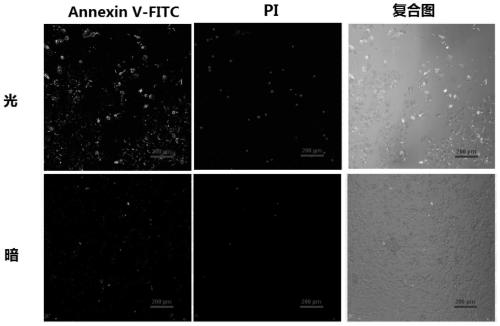
[0041] Antitumor activity experiments and results of the prepared complexes.
[0042] Cells and culture conditions: HeLa (human cervical cancer cell line), SKOV-3 (human ovarian cancer cell line), SKOV-3-ddp (human ovarian cancer cell line cisplatin-resistant cell line), provided by Cancer Hospital, Chinese Academy of Medical Sciences supply. Cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 100 units of penicillin and streptomycin.
[0043] Cytotoxicity Test:
[0044]The cytotoxicity of the complexes was determined by the classical MTT method. Cells were planted on a 96-well plate at a density of 5,000-10,000 cells / well, cultured for 24 hours at 37°C in an environment of 5% carbon dioxide, and then cultured with complexes prepared in Example 1 containing different concentration gradients. 4 hours, at (470nm, 22.5mW / cm 2 ) light intensity for 20 minutes, and then continue to cultivate for 20 hours. After adding 5 mg / ml of MTT (thiazolium blue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com