Preparation method of binary primary alcohol with adjustable molecular weight for bio-based polyurethane
A dibasic primary alcohol, bio-based technology, applied in the field of biomass polyurethane materials, can solve the problems of difficult separation and purification, harsh reaction conditions, low reactivity, etc., and achieve the effect of simple separation and purification, mild reaction process and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
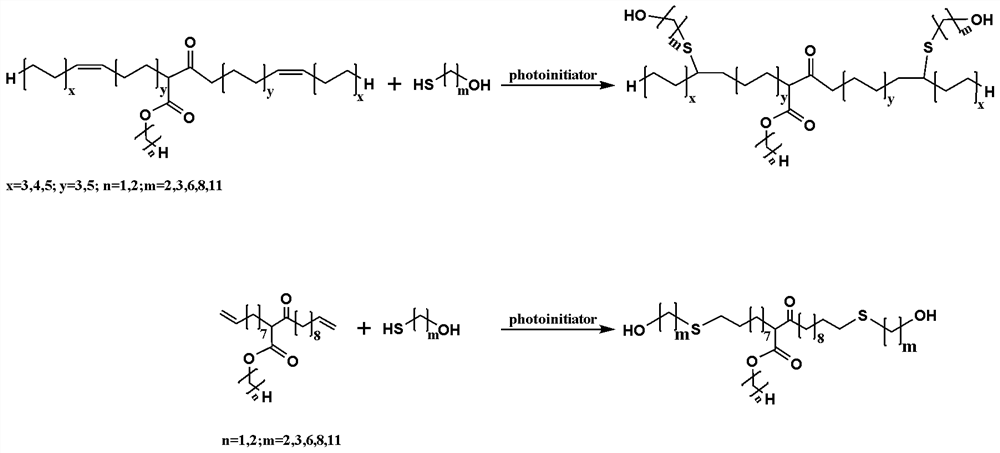
[0023] Preparation of β- keto ester (1): 1 part of sodium hydride in mineral oil after elution added to the reactor, and then were added 8.3 parts of methyl 10- dehydrated undecene, 2.3 parts of dehydrated N, N - dimethylformamide and 4.6 parts of dehydrated dimethyl sulfoxide, nitrogen gas, at an oil bath of 45 ℃ reaction 28 h, after completion of the reaction, the product was slowly poured into dilute hydrochloric acid and the neutralized after the aqueous phase was acidic, and then poured into a separatory funnel and allowed to stand, stratification, the lower aqueous phase was discarded, washed with saturated aqueous sodium chloride was added until the aqueous phase was rendered neutral, collecting the oil phase distilled water under reduced pressure to obtain β - ketoesters, sealed from light, backup;
[0024] Preparation of bio-based diol (2): The above-described parts 1 β- keto ester, 0.42 parts of mercaptoethanol, 0.03 parts of 2,2-dimethoxy-2-phenyl acetophenone was added...
Embodiment 2
[0027] Preparation of β- keto ester (1): 2.8 parts of sodium ethoxide after elution of mineral oil added to the reactor, and then dehydrated successively added 12.3 parts of methyl oleate and 3.8 parts of dehydrated N, N- dimethyl carboxamide, purged with nitrogen, at 65 deg.] C oil bath of the reaction 20 h, after completion of the reaction, the product was slowly poured into dilute hydrochloric acid and the water and the acidic phase, and then poured into a separatory funnel in standing, stratification, the lower aqueous phase was discarded, washed with saturated aqueous sodium chloride was added until the aqueous phase was rendered neutral, the oil phase was collected under reduced pressure to remove water to obtain a β- ketoester, sealed from light, backup;
[0028] Preparation of bio-based diol (2): 2.5 parts of the above-β- keto ester, 1.2 parts of 6-mercapto-1-hexanol, 0.04 parts of 2,2-dimethoxy-2-phenyl benzene ethanone added to a quartz tube, the quartz tube was then pla...
Embodiment 3
[0031](1) Preparation of β-ketorate: 4.7 parts of the eluting mineral oil to the reactor, then add 15.3 parts of the dehydrated mustard, 5 parts dehydrated tetrahydrofuran and 15 dehydrated Diamel sulfoxide, into nitrogen, reacts in a 55 ° C oil supply 24 h, after the reaction is completed, the product is slowly poured into the dilute hydrochloric acid in progress, so that the neutralized water is acidic, and then pour it into points The liquid drain is allowed to stand, layered, discard the lower aqueous phase, add saturated brine to wash until the aqueous phase exhibits neutral, collecting the oil phase under reduced pressure to remove water, obtain keta-ketoate, seal prevent light, standby ;
[0032] (2) Preparation of biological diols: 5 parts of the above keta-ketorate, 3 parts of 11-mercapto-1-elevol, 0.08 parts of residential double methyl ether and 3 parts of dichloromethane (for Dissolved 11-mercapto-1-decolina and anti-rodium double methyl ether) Add in quartz pipe, then...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Iodine value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com