CPA detection primers, detection kit and detection method for enterotoxin B-producing staphylococcus aureus
A technology for detection kits and detection primers, applied in biochemical equipment and methods, methods based on microorganisms, measurement/inspection of microorganisms, etc., can solve the problems of low sensitivity, achieve low detection cost, short time consumption, and reduce false positives. The effect of the probability of a positive result
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The method for detecting and detecting Staphylococcus aureus producing enterotoxin B based on cross-primer constant temperature amplification reaction technology comprises the following steps:
[0043] 1. The reagents used are as follows:
[0044] a. The stripping primers 4s and 5a, the cross-amplification primers 2a1s, and the specific primers 2a and 3a each at a concentration of 10 μM, the primer sequences are as shown in the preceding SEQ ID NO.1-SEQ ID NO.5;
[0045] b.2×Reaction stock solution: Tris-HCl with concentration of 40.0mM, ammonium sulfate of 20.0mM, potassium chloride of 20.0mM, magnesium sulfate of 16.0mM, Tween 20 of 0.2% (v / v), 1.4M Betaine, 10.0mM dNTPs (each) composition;
[0046] c. Bst DNA polymerase (large fragment, NEB company) aqueous solution with a concentration of 8U / μL;
[0047] d. Mixed solution of calcein and manganese chloride: first prepare a calcein solution with a concentration of 50 μM (dissolved in dimethyl sulfoxide); then take 2...
Embodiment 2
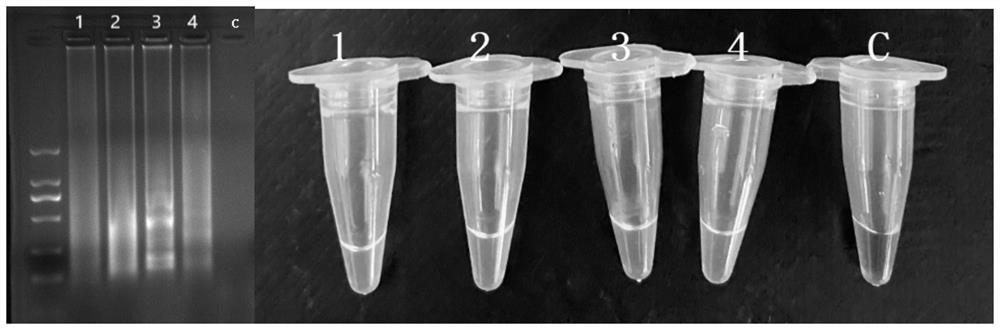
[0059] Genomic DNA of Staphylococcus aureus producing enterotoxin B and non-enterotoxin B Staphylococcus aureus was established according to the reaction system and conditions in Example 1 to establish a cross constant temperature amplification reaction detection method for specificity test;
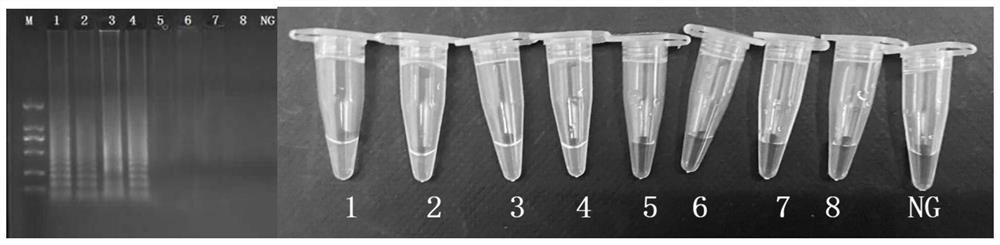
[0060] Staphylococcus aureus that does not produce enterotoxin B is: Salmonella ATCC29629; Salmonella ATCC19585; Salmonella ATCC14028; Salmonella ATCC29629; Listeria monocytogenes ATCC19116; ; Listeria monocytogenes ATCC19113; Pseudomonas aeruginosa ATCC27853; Escherichia coli ATCC43895; Escherichia coli E019 [3] ; Escherichia coli E20 [3] ; Escherichia coli E43 [3] ; Escherichia coli E44 [3] ; Vibrio parahaemolyticus ATCC17802; Vibrio parahaemolyticus ATCC27969; Lactobacillus casei BM-LC14617 [4] ;
[0061] The enterotoxin-producing Staphylococcus aureus genome was set as a positive control, and the nucleic acid-free water was used as a negative control.
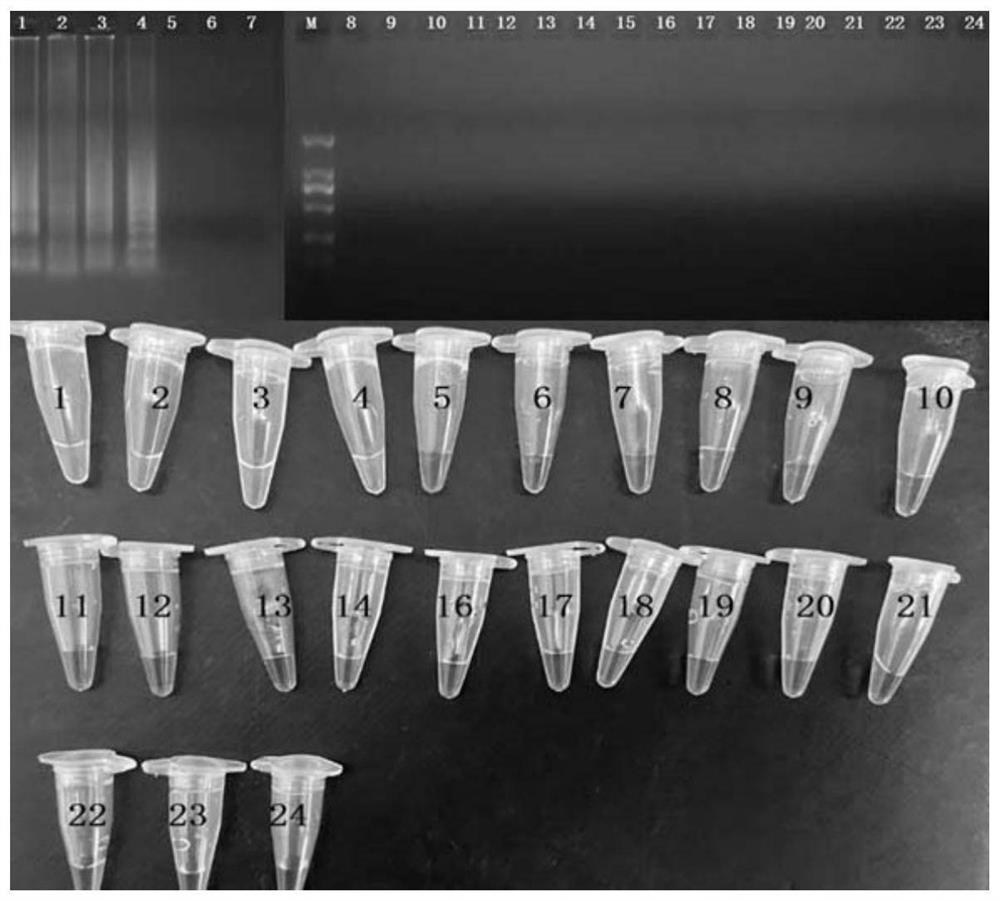
[0062] Carry out 2% agaro...
Embodiment 3
[0065] The sensitivity comparison test for the detection of enterotoxigenic B Staphylococcus aureus by cross constant temperature amplification reaction comprises the following steps:
[0066] Genomic DNA of enterotoxin-producing Staphylococcus aureus was diluted 10-fold, respectively 4.3ng / μL, 430pg / μL, 43pg / μL, 4.3pg / μL, 430fg / μL, 43fg / μL, 4.3fg / μL μL, 430ag / μL. At the same time, a negative control (nucleic acid-free water) was set, and a cross-thermothermal amplification method was constructed according to the reaction system in Example 1, and the amplified product was subjected to 2% agarose gel electrophoresis to determine the sensitivity of the detection method.
[0067] The result is as image 3 Shown is the result graph of the sensitivity test for the detection of enterotoxigenic B Staphylococcus aureus by the cross constant temperature amplification reaction. It can be seen from the figure that trapezoidal bands appear in samples with a DNA concentration higher than...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com