Niraparib preparation method
A compound and catalyst technology, applied in the field of preparation of niraparib, can solve problems such as unfavorable amplification and high cost of platinum, and achieve the effects of reduced synthesis cost, low synthesis cost and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
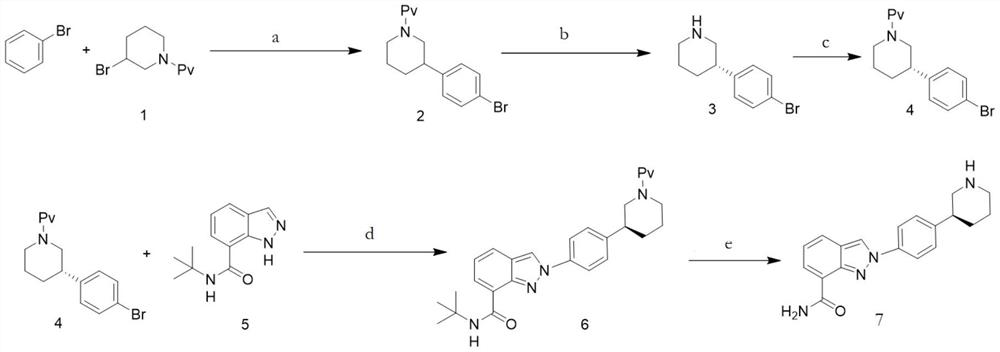
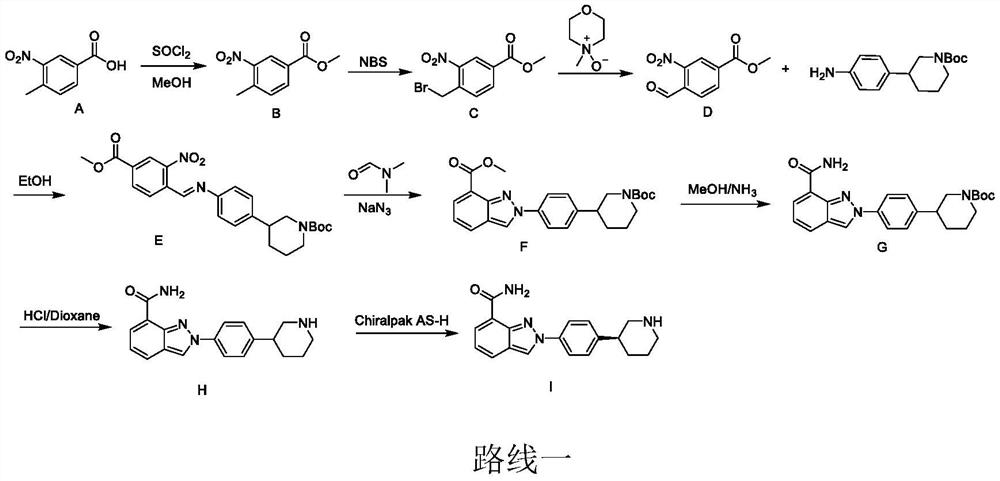
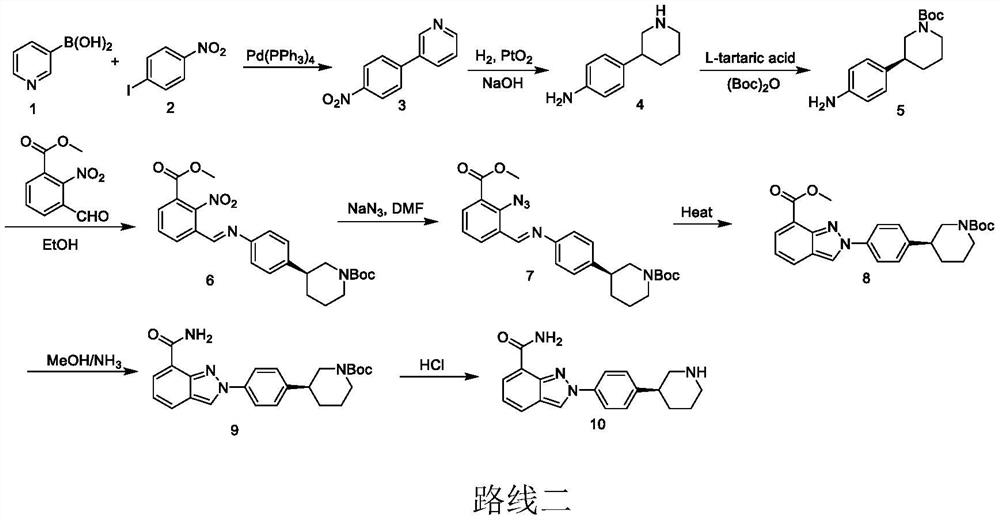
[0031] A preparation method for niraparib, comprising the steps of:
[0032] S1. Dissolve compound 1 and Pd catalyst (0.05eq) in bromobenzene, add potassium formate (1.2eq), and mix to obtain an orange turbid solution;
[0033] Among them, the chemical formula of compound 1 is The Pd catalyst is Pd(PPh 3 ) 4 , Pd(OAc) 2 , Pd 2 (dba) 3 , Pd(dppf)Cl 2 , Pd(PPh 3 )2Cl 2 or PdCl 2 any of the
[0034] S2. Irradiating the orange turbid solution and stirring it until HPLC shows that compound 1 has completely reacted;
[0035] Wherein, the light source used for irradiation is visible light or light with a wavelength of 450nm-480nm or light with a wavelength of 500nm-560nm; the reaction temperature is 20°C-60°C;
[0036] S3. After the reaction is finished, post-processing is carried out to obtain compound 2, which is the key intermediate of niraparib;
[0037] Among them, the post-treatment is specifically: using concentrated treatment to remove the solvent, recrystallizin...
Embodiment 1
[0055] Compound 1 (10.0g, 40.0mmol) was mixed with Pd(PPh 3 ) 4 (0.94g, 0.80mmol) was dissolved in bromobenzene (300mL), and potassium formate (4.20g, 50.0mmol) was added to obtain an orange turbid solution; then, under visible light irradiation, heated to 50°C and stirred for 24h, HPLC showed Compound 1 was completely reacted; after the reaction, washed with saturated sodium chloride (300ml), extracted with ethyl acetate (3╳200mL), combined organic phases, dried with anhydrous sodium sulfate, concentrated, and purified by silica gel column chromatography (petroleum ether / ethyl acetate), the key intermediate of niraparib was obtained as a white solid (6.97g, 54%), and the melting point was 62.5°C-63.1°C.
Embodiment 2
[0057] Compound 1 (10.0g, 40.0mmol) was mixed with Pd(PPh 3 )2Cl 2 (0.56g, 0.80mmol) was dissolved in bromobenzene (300mL), and potassium formate (4.20g, 50.0mmol) was added to obtain an orange turbid solution; then, under visible light irradiation, heated to 50°C and stirred for 24h, HPLC showed The reaction of the first intermediate is complete; after the reaction, wash with saturated sodium chloride (300ml), extract with ethyl acetate (3╳200mL), combine the organic phases, dry with anhydrous sodium sulfate, concentrate, and use a silica gel column Chromatography (petroleum ether / ethyl acetate) yielded the key intermediate of niraparib as a white solid (5.67g, 44%) with a melting point of 62.5°C-63.1°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com