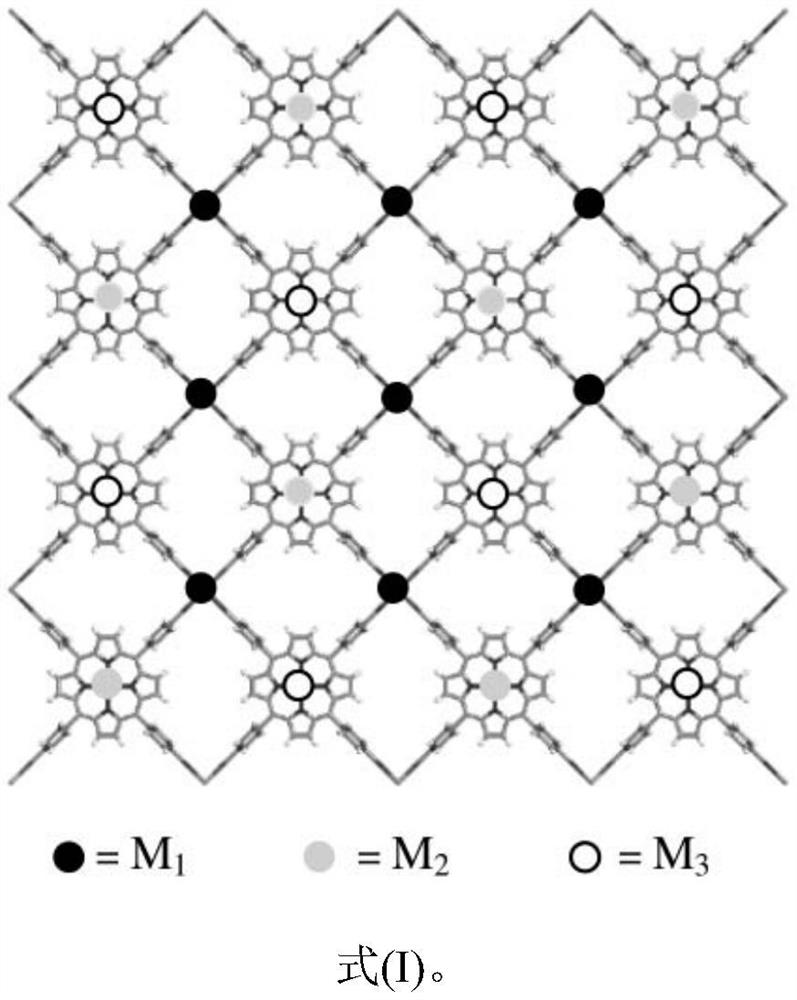
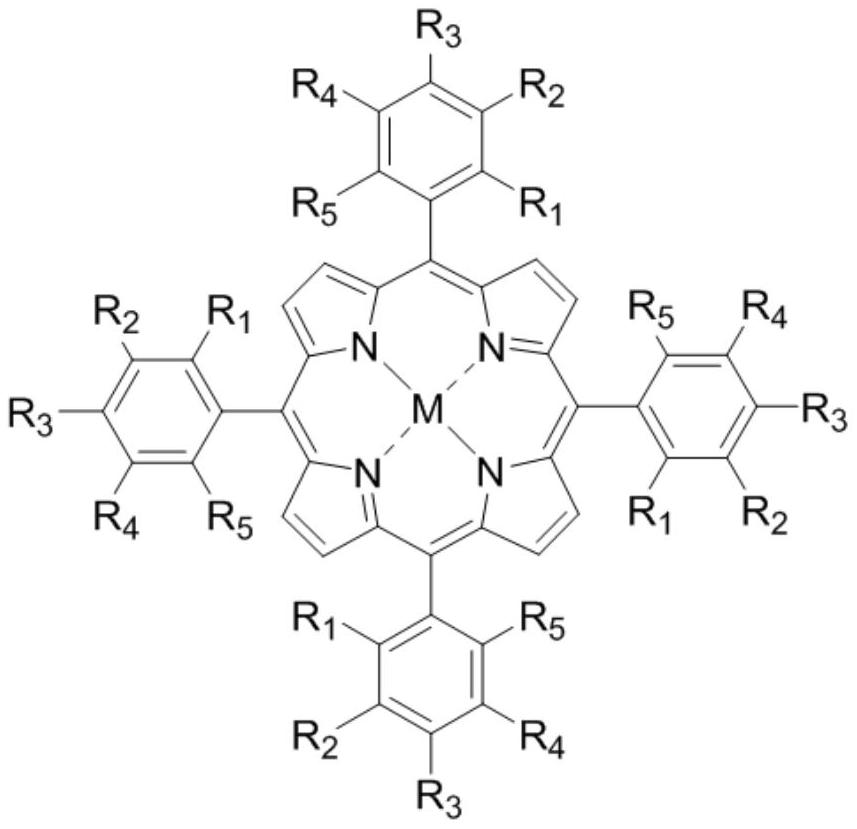
Method for catalytically oxidizing cycloalkane through three-metal-center (CoCuZn) 2D MOFs/ultraviolet light
A catalytic oxidation and trimetallic technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc. Use, reduce the selectivity of cycloalkyl alcohols and cycloalkyl ketones, and increase the uncontrollability of the reaction system to achieve the effects of inhibiting disordered diffusion, high selectivity, and small environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 36
[0026] Embodiment 36 is an enlarged experimental case.
Embodiment 1
[0028]Synthesis of metalloporphyrin trimetallic center (Co&Cu&Zn) 2D MOFs-1: 0.2910 g (1.00 mmol) of cobalt nitrate hexahydrate, 5,10,15,20-tetrakis(4-carboxyphenyl)copper porphyrin (CuTCPP) 0.0426g (0.050mmol), 5,10,15,20-tetrakis (4-carboxyphenyl) porphyrin zinc (ZnTCPP) 0.0427g (0.050mmol) was placed in a 50mL agate ball mill jar, room temperature, 600rpm, ball milling reaction 8.0 h. Stop ball milling once every 1.0h, release the gas in the ball mill tank. After the reaction is complete, transfer the obtained powder to a 10mL centrifuge tube, soak and wash with anhydrous DMF (6×5mL) until the supernatant is clear, soak and wash with acetone (6×5mL) until the supernatant is clear, dry at 40°C for 5.0h, and vacuum at 70°C After drying for 12.0 hours, 0.0662 g of the target product (Co&Cu&Zn)2DMOFs-1 was obtained.
Embodiment 2
[0030] Synthesis of metalloporphyrin trimetallic center (Zn&Cu&Co) 2D MOFs-2: 0.2911 g (1.00 mmol) of zinc nitrate hexahydrate; 5,10,15,20-tetrakis(4-carboxyphenyl)copper porphyrin (CuTCPP) 0.0426g (0.050mmol); 5,10,15,20-tetrakis(4-carboxyphenyl) cobalt porphyrin (CoTCPP) 0.0427g (0.050mmol) was placed in a 50ml agate ball mill jar, room temperature, 600rpm, ball milling reaction 8.0 h. Stop ball milling once every 1.0h, release the gas in the ball mill tank; after the reaction is completed, transfer the powder to a 10mL centrifuge tube, soak and wash in anhydrous DMF (6×5mL) until the supernatant is clear, then soak and wash in anhydrous acetone (6×5mL) 5 mL) until the upper layer was clear, dried at 40°C for 5.0h, and then vacuum-dried at 70°C for 12.0h to obtain 0.0584g of the target product (Zn&Cu&Co)2DMOFs-2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com