Antigen epitope peptide of beta-lactoglobulin, complete antigen, antibody and method for determining residual quantity of beta-lactoglobulin
A technology of lactoglobulin and complete antigen, which is applied in the field of biotechnology and food, can solve the problems such as the difficulty of residual detection of β-lactoglobulin antigen, and achieve the effect of solving the problem of sensitization, accurate detection and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] This embodiment provides a hapten of β-lactoglobulin B cell action epitope AA12-37, and the amino acid sequence of the action epitope peptide is CGAQALIVTQTMKGLDIQKVAGTWYS.
[0065] In the second aspect, this embodiment provides a method for synthesizing the above-mentioned epitope peptide. Specifically, the solid-phase synthesis method is used to link the C-terminal amino acid and Wang resin, and condensation is carried out step by step. After synthesis, the sequence is cut off from the solid phase carrier with strong acid, and the synthesized epitope polypeptide is purified by high performance liquid chromatography, and freeze-dried for subsequent use.
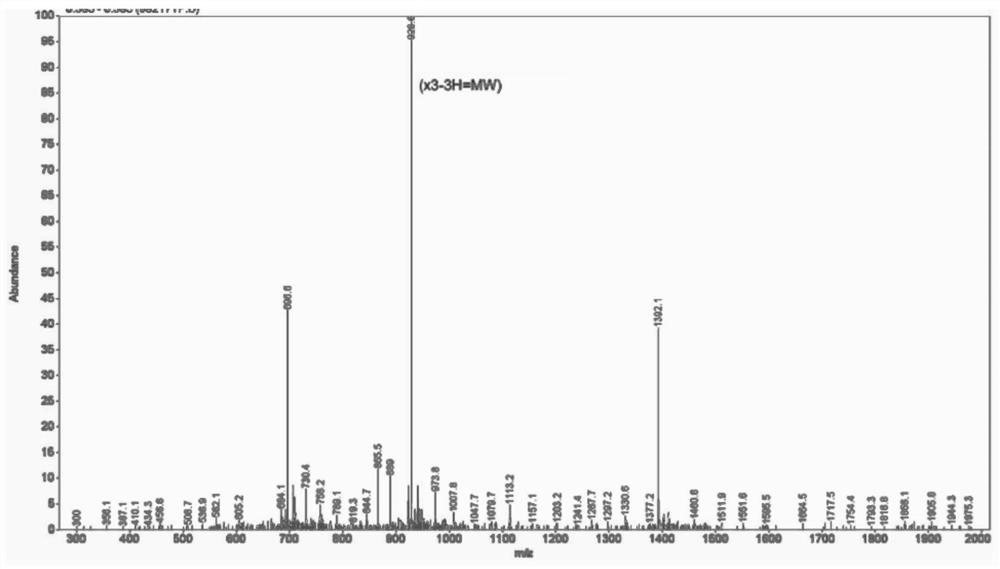
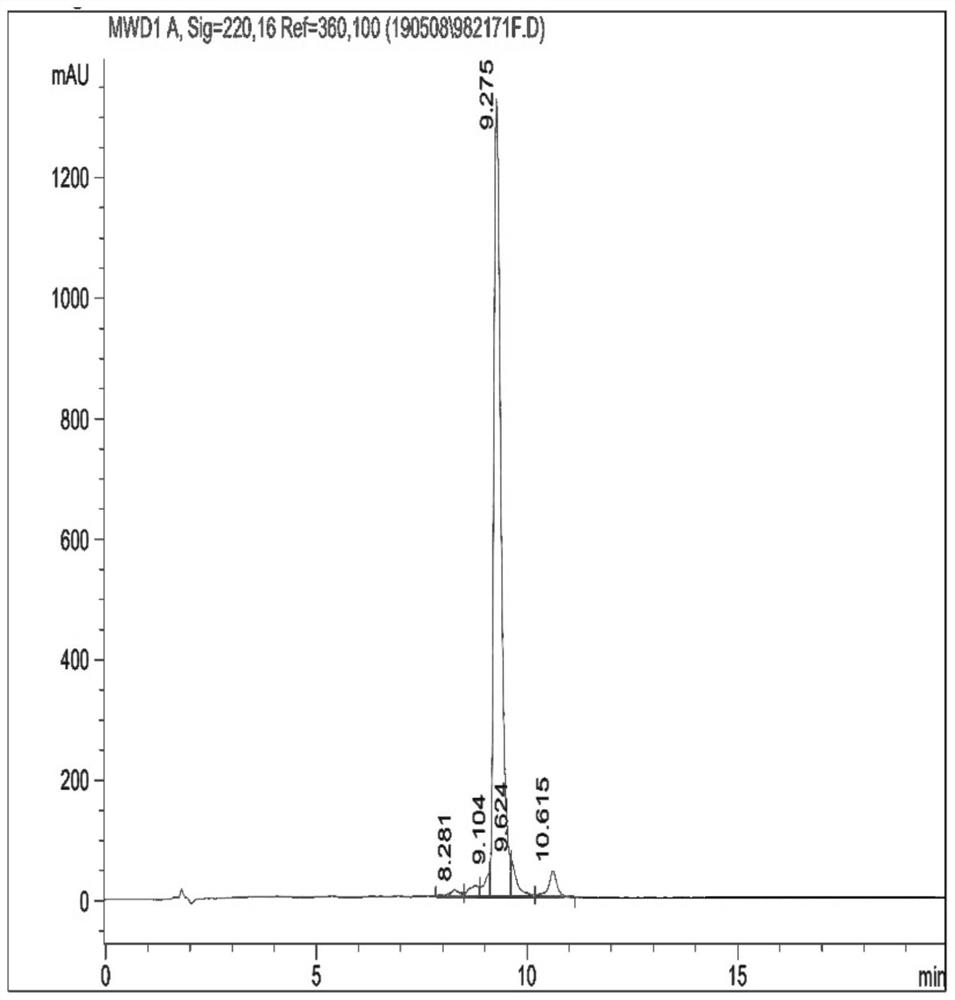
[0066] like figure 1 As shown, the synthesized epitope polypeptide is purified by high performance liquid chromatography, and the purity reaches 92.750%. The relative molecular mass of the epitope peptide was determined by mass spectrometry to obtain figure 2 , the MS spectrum of the sequence CGAQALIVTQTMKGLDIQKVAG...
Embodiment 2
[0068] This example provides a method for synthesizing the complete antigen. Specifically, the purified epitope peptide in Example 1 is coupled with bovine serum albumin (BSA) using the glutaraldehyde method to finally obtain the complete antigen. Bovine serum albumin has stable physical and chemical properties, is not easy to change, has good immune activity, is cheap and easy to obtain, and contains many free amino groups, and has good solubility in different pH values, ionic strengths and organic solvents.
[0069] To identify the complete antigen, specifically, the synthetic epitope BSA and the complete antigen were scanned at the full wavelength of 190-1100nm respectively, and the carrier protein BSA and the synthetic polypeptide had maximum absorption peaks at 210nm and 200nm respectively. The complete antigen has the characteristic absorption peaks of the two at 190-1100nm and there is a certain spectral superposition phenomenon. Therefore, it can be judged that the pre...
Embodiment 3
[0071] This example provides a monoclonal antibody.
[0072] In this example, the complete antigen prepared in Example 2 was used to immunize BALB / c mice. After 4 times of immunization, cell fusion, cell selection, and subcloning were performed several times, and these cells with high activity were injected into BALB / c mice. Intraperitoneal culture of mice, followed by ascitic fluid collection, monoclonal antibody was obtained and purified.
[0073] After obtaining the above monoclonal antibody, in this embodiment, the titer of the monoclonal antibody was determined by an indirect enzyme-linked immunosorbent immunoassay (ELISA) method.
[0074] The antigen coating concentration is 5μg / mL, and the monoclonal antibody is 1:5000, 1:10000, 1:20000, 1:40000, 1:80000, 1:160000, 1:320000, 1:640000, 1:1280000 multiple dilutions.
[0075] The specific determination method of monoclonal antibody potency is as follows:
[0076] ① Antigen coating: Add the diluted antigen to the microti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com