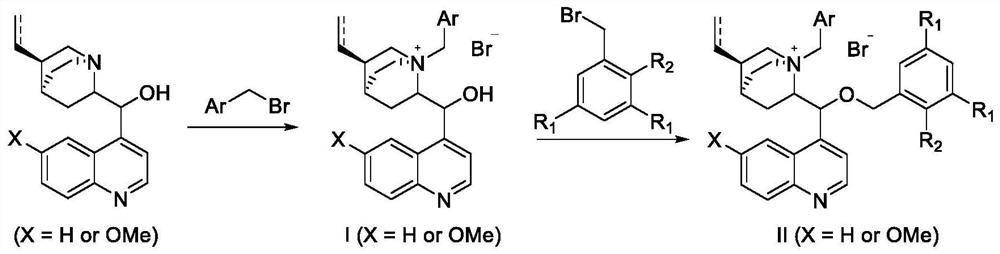
Large-steric-hindrance chiral quaternary ammonium salt phase transfer catalyst derived from quinine and synthesis method thereof
A technology of phase transfer catalyst and cinchona base, which is applied in organic chemical methods, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problems of low yield and poor stability, and achieve the goal of preparing The method is simple, the cost is low, and the effect of using less catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of catalyst C3(Ar 2 Be 3,5-di-tert-butylphenyl), its synthetic method comprises the following steps:
[0041]
[0042] Among them, Ar 2 For 3,5-di-tert-butylphenyl.
[0043] (1) Synthesis of 3,5-di(3,5-di-tert-butylphenyl)benzaldehyde: under nitrogen protection, 3,5-dibromobenzaldehyde (1.57g, 6mmol), 3,5-dibromobenzaldehyde tert-butylphenylboronic acid (12mmol) and Pd(PPh 3 ) 4 (69.3mg, 0.06mmol was dissolved in 12mLTHF (remove oxygen by bubbling). After stirring at room temperature until the solid reactant was completely dissolved, add Na 2 CO 3 (3.8 g, 36 mmol) in water (6 mL). The reaction system was heated to reflux temperature. After the reaction was completed, the mixture was filtered through celite. The filtrate was extracted 3 times with ethyl acetate. The organic phases were combined and dried over anhydrous sodium sulfate. Concentrated under reduced pressure and purified by silica gel column chromatography to obtain 3,5-bis(3,5-di-ter...
Embodiment 2
[0050] Preparation of Catalyst C4
[0051]
[0052] (1) Dissolve cinchona base (118 mg, 0.4 mmol) in toluene (6 mL), add 3,4-difluorobenzyl bromide (160 mg, 0.52 mmol), and heat to reflux for 2 hours. After the reaction was completed, N-3,4-difluorobenzyl gallonine quaternary ammonium salt I (178 mg, 89%) was obtained by silica gel column chromatography;
[0053] (2) Dissolve N-3,4-difluorobenzyl chicken sodium quaternary ammonium salt Ⅰ (178mg, 0.356mmol) in dichloromethane (6mL), add 3,5-bis(3,5-di-tert-butyl phenyl) benzyl bromide (594mg, 1.07mmol) and 50% KOH aqueous solution (100mg, 1.78mmol), reacted and separated by column chromatography to obtain N-3,4-difluorobenzyl-O- 3,5-Bis(3,5-di-tert-butylphenyl)benzyl chicken sodium base-derived quaternary ammonium catalyst C4 (267 mg, 75%).
Embodiment 3
[0055] Preparation of Catalyst C5
[0056]
[0057] (1) Synthesis of 2-bromo-3,5-di-tert-butylbenzyl bromide: 3,5-di-tert-butyltoluene (409 mg, 2.0 mmol) was dissolved in anhydrous CH 3 CN, add FeCl successively at room temperature 3 (65 mg, 0.4 mmol) and NBS (374 mg, 2.1 mmol). The reaction was heated to 82°C and stirring was continued for 4 hours. After the reaction, the resulting solution was cooled to room temperature, and the solvent was removed by rotary evaporation.
[0058] The crude product was purified by silica gel column chromatography using petroleum ether as the eluent to obtain 2-bromo-3,5-di-tert-butyltoluene with a yield of 89%. Dissolve 2-bromo-3,5-di-tert-butyltoluene (468mg, 2.0mmol) in cyclohexane, add NBS (324mg, 2.06mmol) and BPO (13mg, 0.066mmol) successively at room temperature, and heat to 80 °C, reflux for 4 hours. After the reaction was completed, the solvent was removed by rotary evaporation. The crude product was purified by silica gel co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com