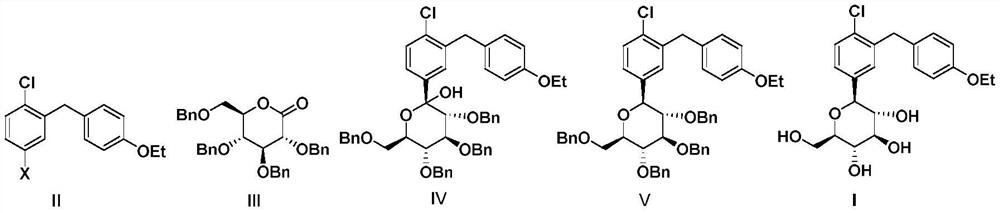
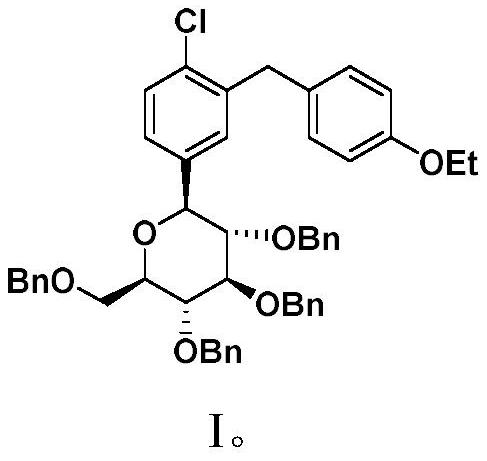
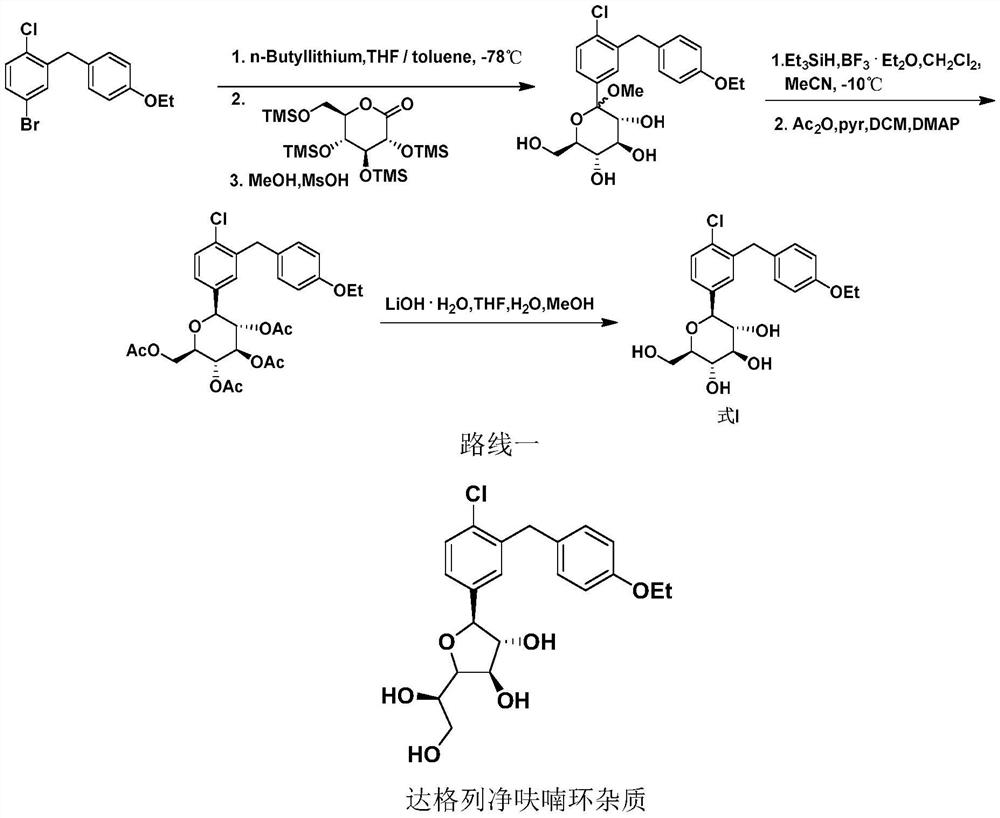
Preparation method of dapagliflozin
A compound and reaction technology, applied in the field of preparation of dapagliflozin, can solve the problems of poor production safety, long process steps, unfriendly environment, etc., and achieve the advantages of simple operation, few synthesis steps, and reduction of post-processing steps and waste generation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (1) (3R,4S,5R,6R)-3,4,5-tris(benzyloxy)-6-((benzyloxy)methyl)-2-(4-chloro-3-(4- Synthesis of Ethoxybenzyl)phenyl)tetrahydro-2H-pyran-2-ol (Ⅳ)
[0058] Dissolve 17.45g (53.6mmol) of the compound shown in formula II-1 in 107mL of toluene-tetrahydrofuran (V(toluene):V(tetrahydrofuran)=2:1) to obtain a mixed solution A; 24mL of 2.5mol / L n-butyl The n-hexane solution of lithium was used as solution B; Tetrahydro-2H-pyran-2-one (III) was dissolved in 75 mL of toluene to obtain a mixed solution C.
[0059] Under the protection of nitrogen, the mixed solution A and the solution B are respectively pumped into the first micro-mixer, and after being fully mixed, they are reacted in the first micro-channel reactor to obtain a reaction effluent containing an aryl lithium compound. Among them, the injection rate of A is 2.28mL / min, the injection rate of B is 0.5mL / min, the reaction volume of the first microchannel of the microchannel reaction device is 42mL, the reaction temperat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com