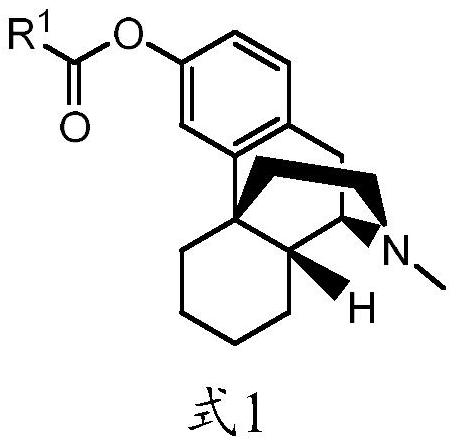
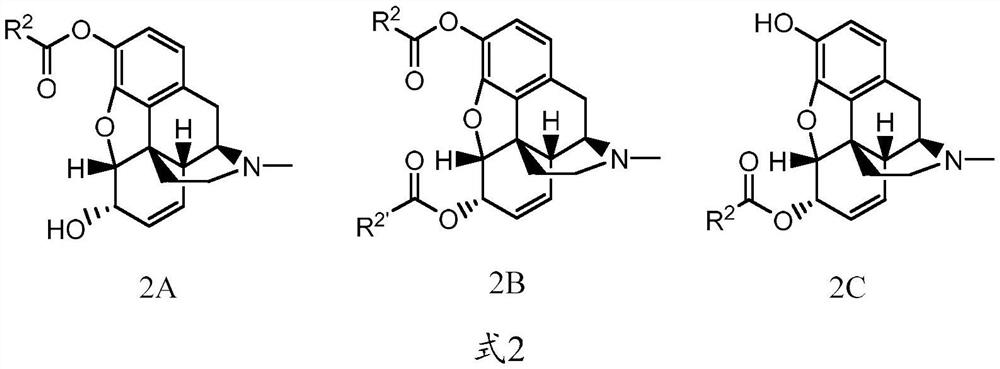
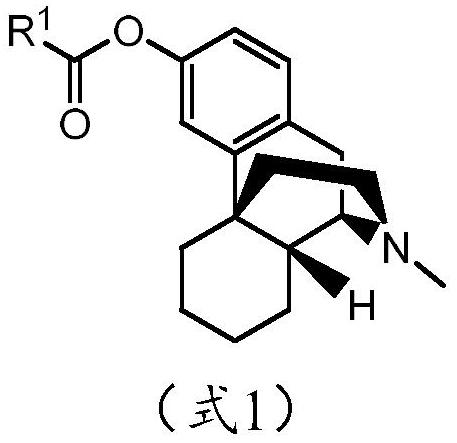
Abuse-resistant long-acting release opioid prodrugs
A pharmaceutical and compound technology applied in the field of anti-abuse long-acting release opioid prodrugs, which can solve the problems of limited success of formulation technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Embodiment 1—synthesis of levorphanolate prodrug
[0108]
[0109] Exemplary Procedure for Preparation of Levorphanol Palmitate (n=14). Fatty acid (palmitic acid) and sulfonyl chloride (>10 molar equivalents) were added to a dry round bottom flask. The mixture was refluxed at 85° C. for 2 hours in an oil bath. The liquid sulfonyl chloride was removed under vacuum using a rotary evaporator and further using an oil pump. anhydrous CH 2 Cl 2 Add to the mixture. The added solvent was then removed under vacuum using a rotary evaporator and further using an oil pump. Will add and remove anhydrous CH 2 CH 2 The procedure was repeated three times to ensure that residual sulfonyl chloride was removed. The obtained pale yellow crystals were used in the next reaction without further purification.
[0110] Levorphanol tartrate salt (1.0 molar equiv) and triethylamine (4.0 molar equiv) were dissolved in anhydrous CH in a round bottom flask 2 Cl 2 middle. Place the flask...
Embodiment 2
[0117] Embodiment 2—Synthesis of morphine ester prodrug
[0118]
[0119] According to the method described in Example 1, using morphine chloride salt instead of compound 5-8, morphine monoesters with n being 12, 14, 16 and 20 were prepared respectively. Morphine diesters can also be prepared by a similar method, except that 2 equivalents or more of the acid chloride are used. Other monoesters can be prepared similarly, although protection / deprotection methods can be used to increase reaction yields.
Embodiment 3
[0120] Example 3 - Stability Study Under Tampering Conditions
[0121] Prodrugs were subjected to common tampering conditions including 1.0 M baking soda (pH = 8.3), vinegar (5% acetic acid, pH = 2.5) and vodka (40% alcohol) at 80 °C, and chlorine gas at 25 °C and hydrogen peroxide. The final incubation mixture contained 10 [mu]M test compound in a final volume of 0.5 mL of tamper medium. The prodrug is added to start the incubation. At 0 min, 30 min, and 60 min, 0.05 mL aliquots were removed from the incubation mixture, quenched with 0.15 mL methanol, and placed on ice. Aliquots were removed for analysis. Concentrations of prodrug and parent drug were analyzed by LC-MS / MS to compare prodrug stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com