Opiates painkiller and opiate receptor antagonist-containing medicinal composition
A composition and drug technology, applied in the field of medicine, can solve the problem of no improvement in the analgesic activity of analgesics, achieve the effect of reducing adverse reactions and enhancing analgesic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
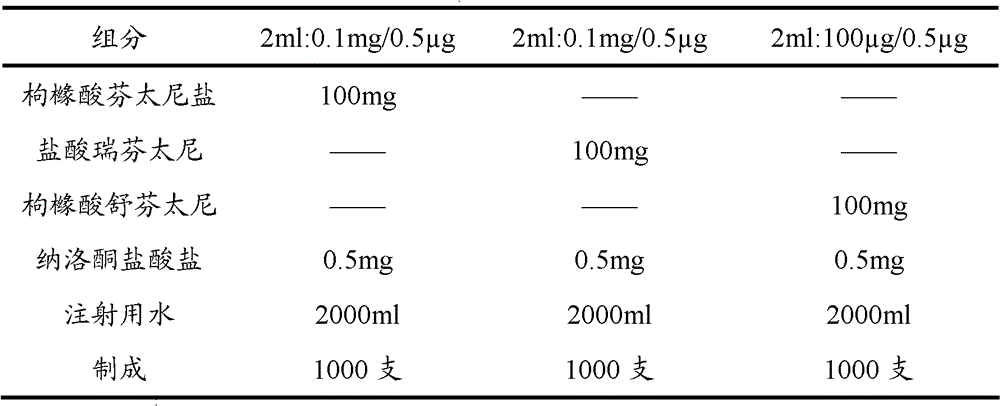
[0063] Embodiment 1: Opioid analgesic and naloxone hydrochloride injection
[0064] prescription:
[0065]
[0066] Preparation:
[0067] Take 80% of the prescribed amount of water for injection in the batching tank, add the main drug of the prescribed amount, stir until it is completely dissolved, adjust the pH value with sodium hydroxide solution or hydrochloric acid solution, and adjust the volume to the full amount. After the pH value is qualified, add 0.1% Activated carbon was stirred at room temperature for 45 minutes, then decarbonized by coarse filtration, and finely filtered by a 0.22 μm microporous membrane. 2 Potting, sterilizing, light inspection, and packaging.
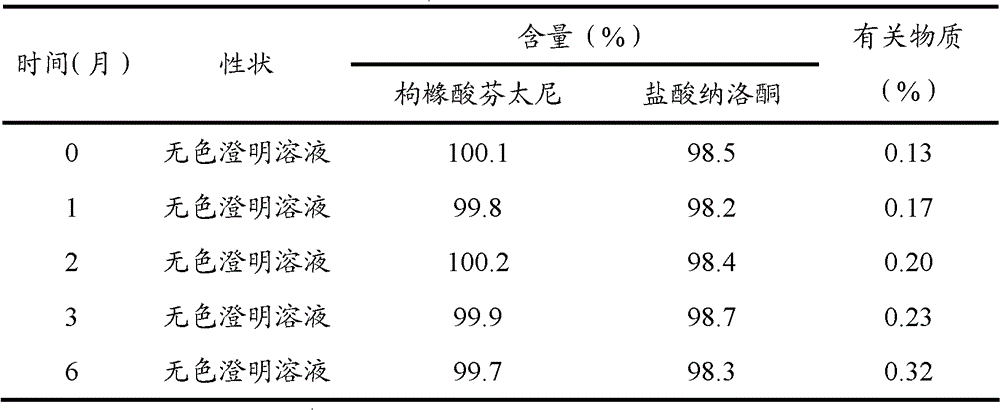
[0068] Preparation stability investigation test results:
[0069] The fentanyl citrate / naloxone hydrochloride injection prepared according to the above-mentioned prescription process was subjected to accelerated test and long-term test investigation. The results are shown in Table 1 and Table 2.
...
Embodiment 2
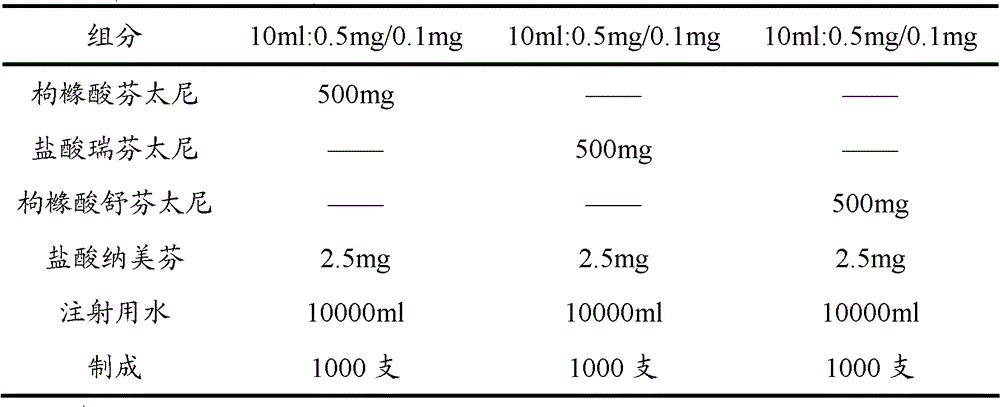
[0073] Embodiment 2: Opioid analgesic and nalmefene hydrochloride injection
[0074] prescription:
[0075]
[0076] Preparation:
[0077] Take 80% of the prescribed amount of water for injection in the batching tank, add the main drug of the prescribed amount, stir until it is completely dissolved, adjust the pH value with sodium hydroxide solution or hydrochloric acid solution, and adjust the volume to the full amount. After the pH value is qualified, add 0.1% Activated carbon was stirred at room temperature for 45 minutes, then decarbonized by coarse filtration, and finely filtered by a 0.22 μm microporous membrane. 2 Potting, sterilizing, light inspection, and packing.
Embodiment 3
[0078] Example 3: Generic Tablets of Opioid Analgesics and Naltrexone Hydrochloride
[0079] prescription:
[0080]
[0081] Preparation:
[0082] Premix the opioid analgesics, naloxone hydrochloride, and silicon dioxide in the amounts listed in the above table through 80 mesh for 3 times, add 5 times the amount of lactose for mixing, and then add 10 times the amount of lactose each time Mix, finally add magnesium stearate as a lubricant, compress after total mixing, and pack to get final product.
[0083]Taking fentanyl and naloxone tablets (100μg / 0.5mg) as examples, the dissolution rate was measured. The tablets were tested for drug release by the paddle method in 250 ml of purified water with a paddle speed of 50 rpm. The media samples were taken out periodically, and the samples were analyzed by high performance liquid chromatography, and the dissolution results were listed in Table 3.
[0084] Table 2 Dissolution results of fentanyl and naloxone tablets (100 μg / 0.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com