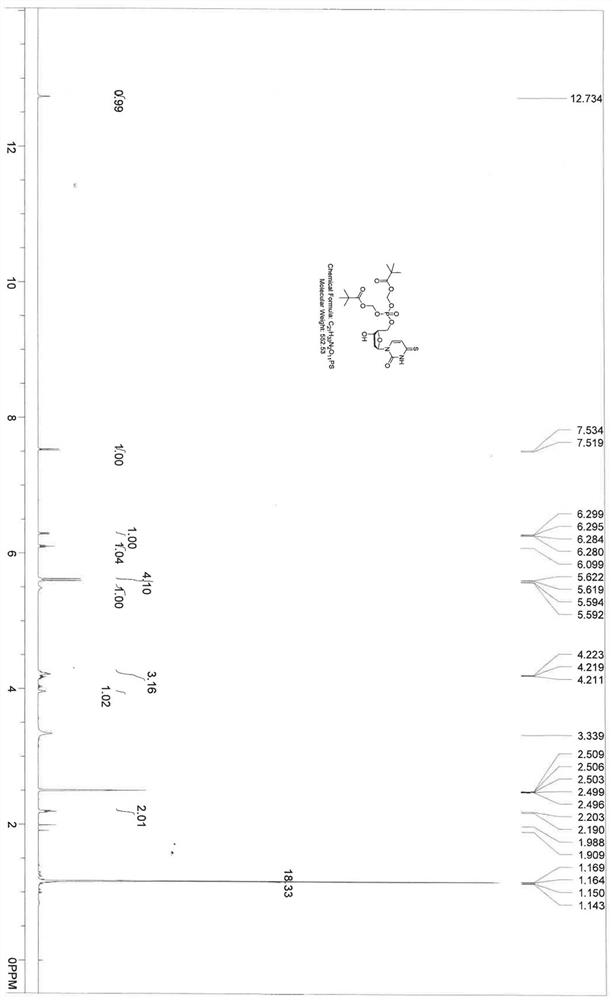
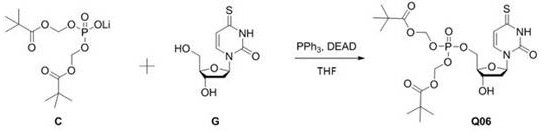
4-thiouracil deoxynucleoside phosphate and application thereof to antiviral drugs
A technology of deoxynucleoside phosphate and thiouracil, which is applied in the direction of antiviral agents, phosphorus organic compounds, sugar derivatives, etc., can solve the problems of weak drug efficacy, drug resistance, and relapse after drug withdrawal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the synthesis of compound B
[0035] .
[0036] 1) Compound A (25 g, 178.48 mmol) was dissolved in acetonitrile (250 mL), the reaction mixture was replaced with argon and protected by argon, and then trimethyl phosphate (107.5 g, 713.93 mmol), Sodium iodide (80 g, 535.45 mmol) and 4A molecular sieves were added completely, and the temperature of the reaction liquid oil bath was raised to 90°C and stirred overnight. The reaction solution was cooled to room temperature, filtered with diatomaceous earth, and the filtrate was concentrated under reduced pressure to obtain a crude product, which was separated by silica gel column chromatography (petroleum ether / ethyl acetate=15 / 1~5 / 1) to obtain compound B (50.7 g, colorless and transparent Oil, yield: 65%).
[0037] 2) Compound A (11.2 g, 79.96 mmol) was dissolved in acetonitrile (100 mL), the reaction mixture was replaced with argon and protected by argon, and then trimethyl phosphate (41.15 g, 279.86 mmol)...
Embodiment 2
[0038] Embodiment 2: the synthesis of compound C
[0039] .
[0040] 1) Compound B (50.7 g, 115.11 mmol) was dissolved in acetonitrile (250 mL), and then lithium bromide (10 g, 115.11 mmol) was added to the reaction solution, the reaction mixture was replaced with argon, and the temperature of the oil bath was raised Reaction at 90°C for 16h. The reaction solution naturally cooled to room temperature, and a large amount of solids precipitated out. After filtration, the filter cake was washed with petroleum ether, and the filter cake was dried to obtain compound C (26.6 g, white solid, yield: 71.9%).
[0041] 2) Compound B (23.9 g, 54.27 mmol) was dissolved in acetonitrile (150 mL), and then lithium bromide (4.7 g, 54.27 mmol) was added to the reaction solution, and the reaction mixture was replaced by argon under argon protection, and the oil bath The temperature was raised to 90°C for 16h. The reaction solution was naturally cooled to room temperature and concentrated un...
Embodiment 3
[0043] 1) Synthesis of Compound E
[0044] .
[0045] Compound D (5 g, 21.91 mmol) and acetic anhydride (4.92 g, 48.2 mmol) were dissolved in pyridine (50 mL), the reaction mixture was replaced with argon and protected by argon, and stirred at room temperature for 16 h. The reaction solution was directly concentrated under reduced pressure, diluted with dichloromethane (200 mL), washed the organic phase with saturated citric acid (80 mLx2), washed once with saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain the compound E (7.57 g, white solid).
[0046] 2) Synthesis of Compound E
[0047] .
[0048] Compound D (5 g, 21.91 mmol) and acetic anhydride (4.92 g, 48.2 mmol) were dissolved in acetonitrile (50 mL), the reaction mixture was replaced with argon and protected by argon, stirred for 10 min, and triethylamine ( 4.88 g, 48.2 mmol), stirred at room temperature for 16h. The reaction soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com