Non-noble metal Ni-based catalyst, preparation method thereof and method for preparing cyclopentane through cyclopentadiene hydrogenation
A non-precious metal, cyclopentadiene technology, used in metal/metal oxide/metal hydroxide catalysts, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of easy deactivation and high cost, and achieve the reuse rate High, high selectivity and yield, reducing the effect of self-agglomeration coke
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
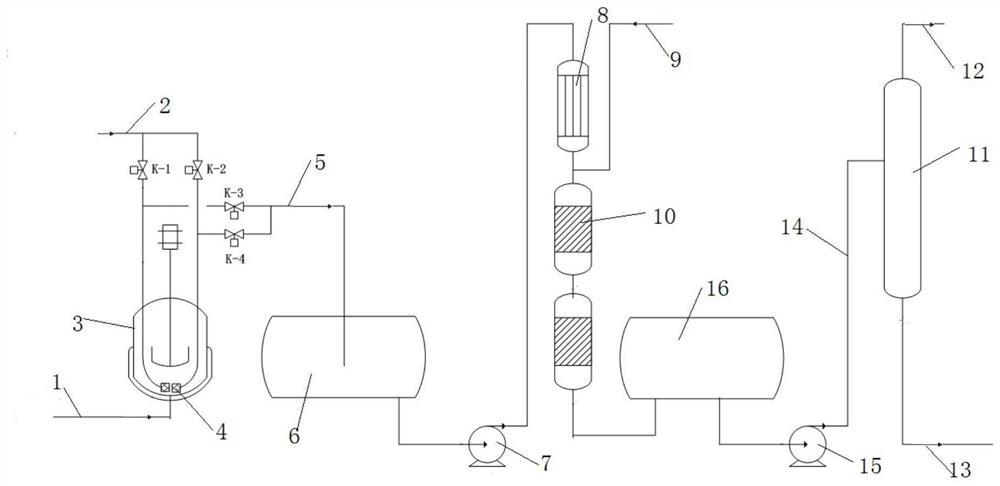
[0031] The present invention provides a kind of preparation method of non-precious metal Ni base catalyst on the other hand, comprises the following steps:
[0032] (1) Ni active metal component precursor, metal additive component precursor and nitrogen-containing organic acid are loaded on the carrier by impregnation method, and catalyst precursor I is obtained after drying;
[0033] (2) pyrolyzing the catalyst precursor I in an inert atmosphere to obtain the catalyst precursor II;
[0034] (3) The catalyst precursor II is contacted with a reducing gas under the reduction reaction condition that the combined nickel is reduced to elemental nickel.
[0035]In the present invention, nitrogen-containing organic acid is added in the loading process of the metal active component and the metal additive component, and firstly pyrolyzed under an inert atmosphere, and then reduced, so that the obtained catalyst can hydrogenate cyclopentadiene In the preparation of cyclopentane, the se...
Embodiment 1
[0075] Preparation of non-noble metal Ni-based catalyst:
[0076] Prepare nickel nitrate (Ni(NO) containing 0.35g Ni 3 ) 2 ) aqueous solution, prepare chromium nitrate (Cr(NO 3 ) 3 ) aqueous solution, mixing the two, and then adding 2.0 g of ethylenediaminetetraacetic acid to obtain 100 mL of impregnating solution, immersing 10 g of mordenite carrier in the above impregnating solution, and evaporating to dryness at 80° C. to obtain catalyst precursor I;
[0077] Catalyst precursor I was pyrolyzed at 450° C. for 12 hours in a nitrogen atmosphere (nitrogen concentration: 30% by volume) to obtain catalyst precursor II;
[0078] The catalyst precursor II was reduced at 400°C for 4 hours in a hydrogen atmosphere (the concentration of hydrogen was 15% by volume), and then the catalyst obtained after reduction was shaped into a clover-shaped catalyst, and the shaped catalyst was dried to obtain the non-noble metal Ni base catalyst.
[0079] After testing, the components in the n...
Embodiment 2
[0093] Preparation of non-noble metal Ni-based catalyst:
[0094] Prepare nickel nitrate (Ni(NO) containing 0.45g Ni 3 ) 2 ) aqueous solution, prepare chromium nitrate (Cr(NO 3 ) 3 ) aqueous solution, mixing the two, and then adding 1.0 g of ethylenediaminetetraacetic acid to obtain 100 mL of impregnating solution, immersing 10 g of mordenite carrier in the above impregnating solution, and evaporating to dryness at 80° C. to obtain catalyst precursor I;
[0095] Catalyst precursor I was pyrolyzed at 550° C. for 24 hours in a nitrogen atmosphere (nitrogen concentration: 30% by volume) to obtain catalyst precursor II;
[0096] The catalyst precursor II was reduced at 350°C for 2 hours in a hydrogen atmosphere (the concentration of hydrogen was 30% by volume), and then the catalyst obtained after reduction was shaped into a clover-shaped catalyst, and the shaped catalyst was dried to obtain the non-noble metal Ni base catalyst. The content of each component of the obtained c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com