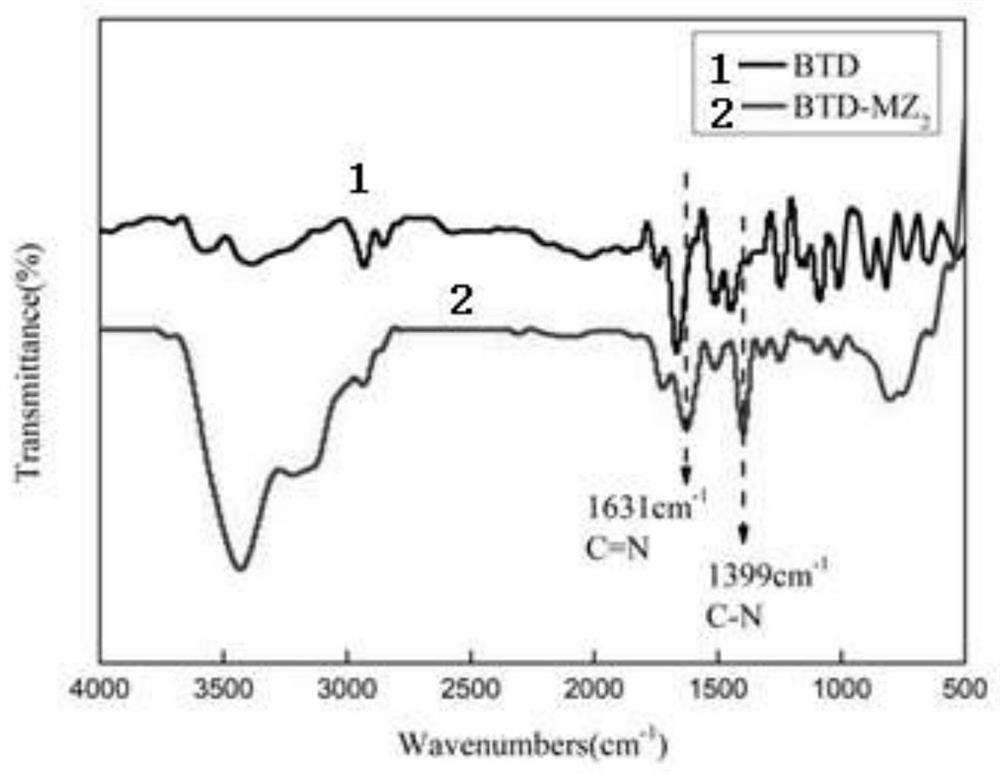
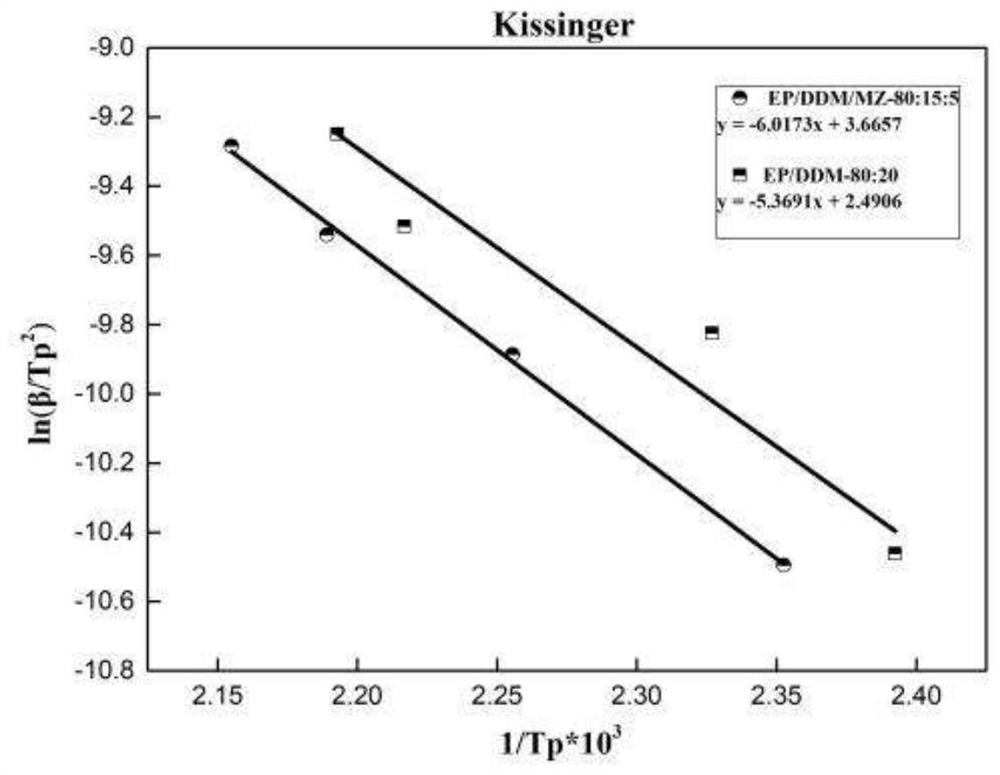
Benzothiadiazole modified imidazole compound as well as preparation method and application thereof
A technology of benzothiadiazoles and benzothiadiazoles, which is applied in the field of benzothiadiazole-modified imidazole compounds and its preparation, can solve the problem of high curing temperature of benzoxazines, which is unfavorable to actual industrial production and affects Material physical properties and other issues, to achieve the effect of easy to obtain raw materials, improve latent properties, and overcome short pot life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The invention provides a kind of preparation method of benzothiadiazole modified imidazole compound, comprising:
[0039] Under nitrogen atmosphere, under the effect of catalyst, solvent and acid-binding agent, react the imidazole of formula 2 structure and the 4,7-dibromo-2 of formula 3 structure, 1,3-benzothiadiazole compound, The solvent is preferably selected from one of N,N-dimethylformamide, dimethyl sulfoxide or N,N-dimethylacetamide; the catalyst is preferably cuprous iodide, and the The acid-binding agent is preferably selected from one of anhydrous potassium carbonate, triethylamine, sodium hydroxide or potassium hydroxide, the reaction temperature is preferably 140-155°C, the reaction time is preferably 48-72h, and the reaction ends After that, it is preferably cooled to room temperature, diluted with water, filtered, washed and dried to obtain a crude product, dissolved in a solvent, ultrasonically filtered, the filtrate is rotary evaporated, and dried to ob...
Embodiment 1
[0052] 1. Add 15.03g of 2,1,3-benzothiadiazole, 40.62g of N-bromosuccinimide, and 150ml of concentrated sulfuric acid into a 500ml single-necked flask; after mixing evenly, react the mixed solution in an oil bath at 60°C 3h; after the reaction, cool to room temperature, wash with concentrated sulfuric acid in an ice-water bath, filter and dry to obtain 4,7-dibromo-2,1,3-benzothiadiazole.
[0053] 2. Add 31.38g 4,7-dibromo-2,1,3-benzothiadiazole and excess imidazole to a 500ml three-necked flask to meet the requirements of imidazole and 4,7-dibromo-2,1,3-benzene The molar ratio of thiadiazole is 1:3, 2.474g cuprous iodide, 17.756g anhydrous potassium carbonate, 120ml N,N-dimethylformamide, react at 150℃ for three days under nitrogen atmosphere; after the reaction Cool to room temperature, dilute with water, filter, wash and dry to obtain the crude product, dissolve the crude product in dichloromethane, filter with ultrasonic for 30 minutes, spin the filtrate at 45°C, and dry to...
Embodiment 2
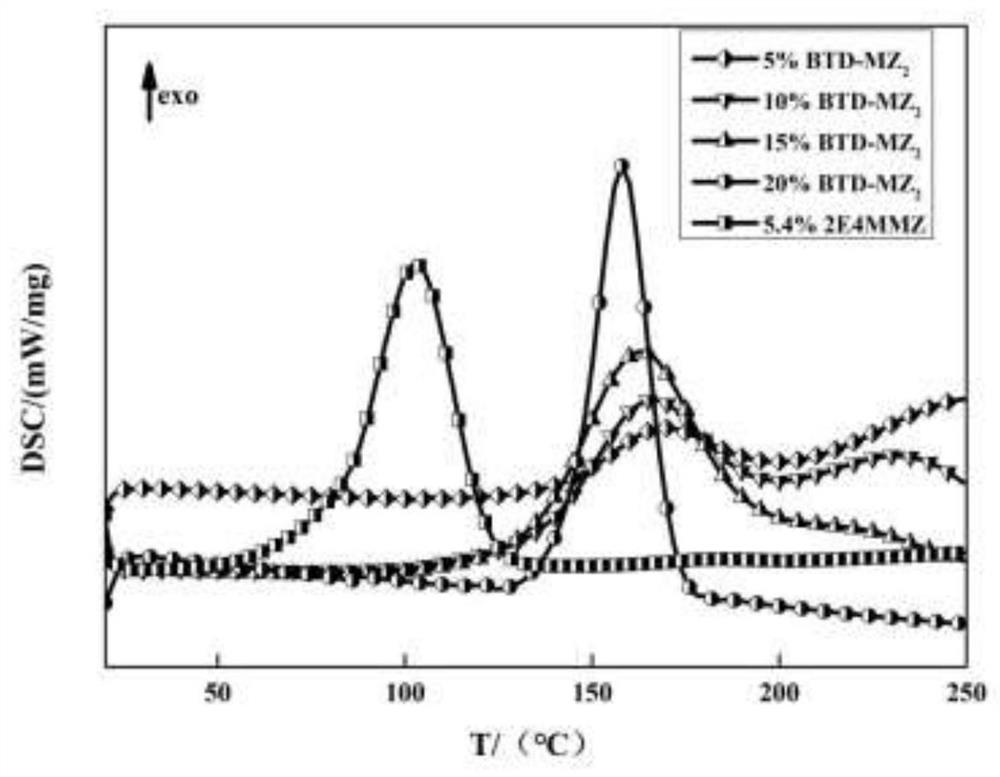
[0059] The preparation method and conditions of this example are the same as in Example 1, the difference is that in application, the benzothiadiazole modified imidazole compound BTD-MZ 2 The mass ratio of epoxy resin and E51 type epoxy resin is 10:100 to obtain a latent epoxy resin one-component curing system. It can be stored for more than 850min at 60°C (such as Figure 4 ), the viscosity remained basically unchanged. According to DSC detection, the exothermic temperature of the initial curing reaction is 145.7°C, the highest peak is at 167.9°C, and the exotherm ends at 190.5°C (such as Figure 5 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com