Epigallocatechin gallate sustained-release capsule and preparation method thereof
A technology of epigallocatechin and gallate, which is applied in the field of epigallocatechin gallate sustained-release capsules and its preparation, can solve the unsolved problems of hygroscopicity and fluidity, and affect the dissolution rate and Curative effect, compliance, poor compliance and other issues, to achieve the effect of prolonging the effective time of action, reducing the fluctuation of blood drug concentration, and stabilizing the quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
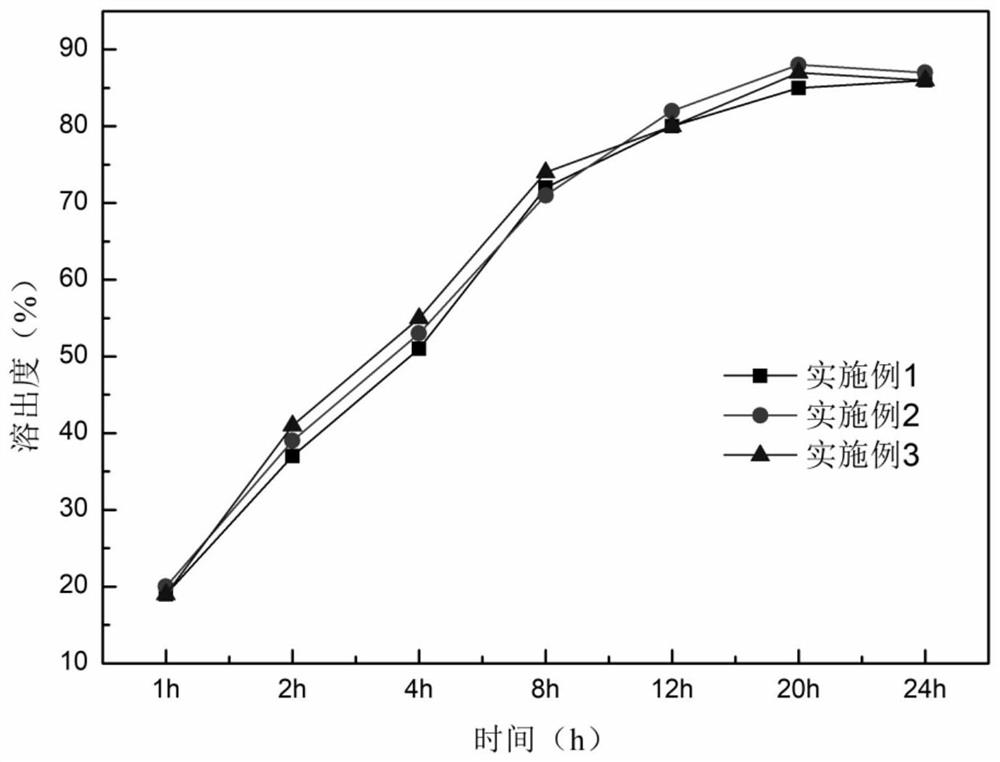
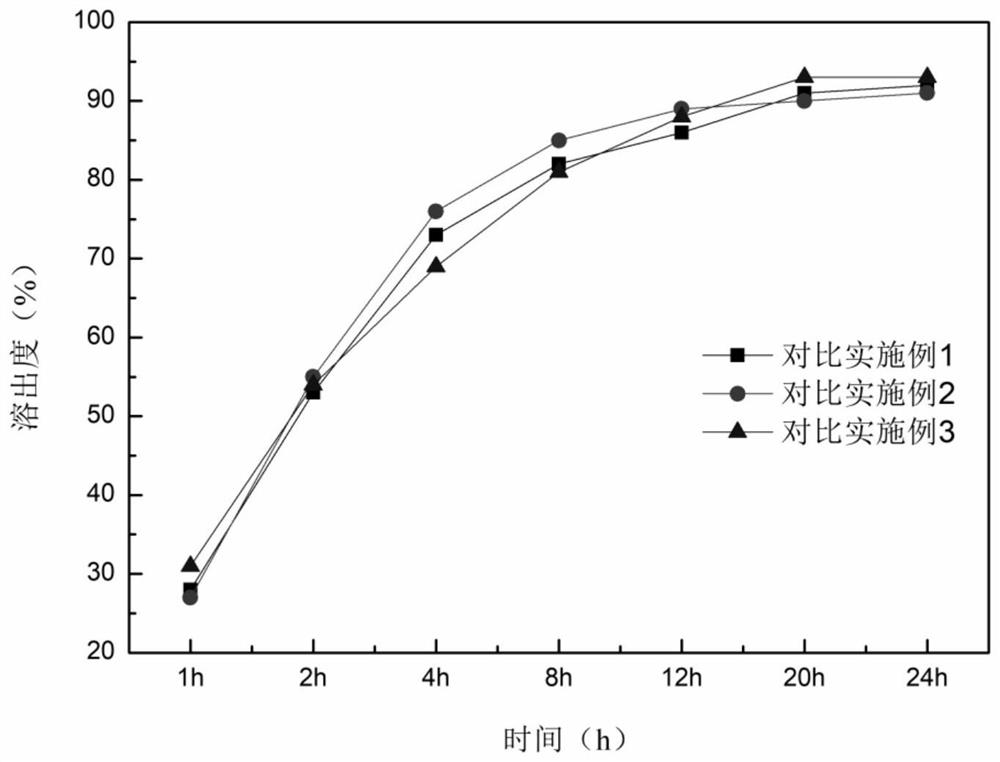
Embodiment 1
[0037]
[0038] 2. Preparation method
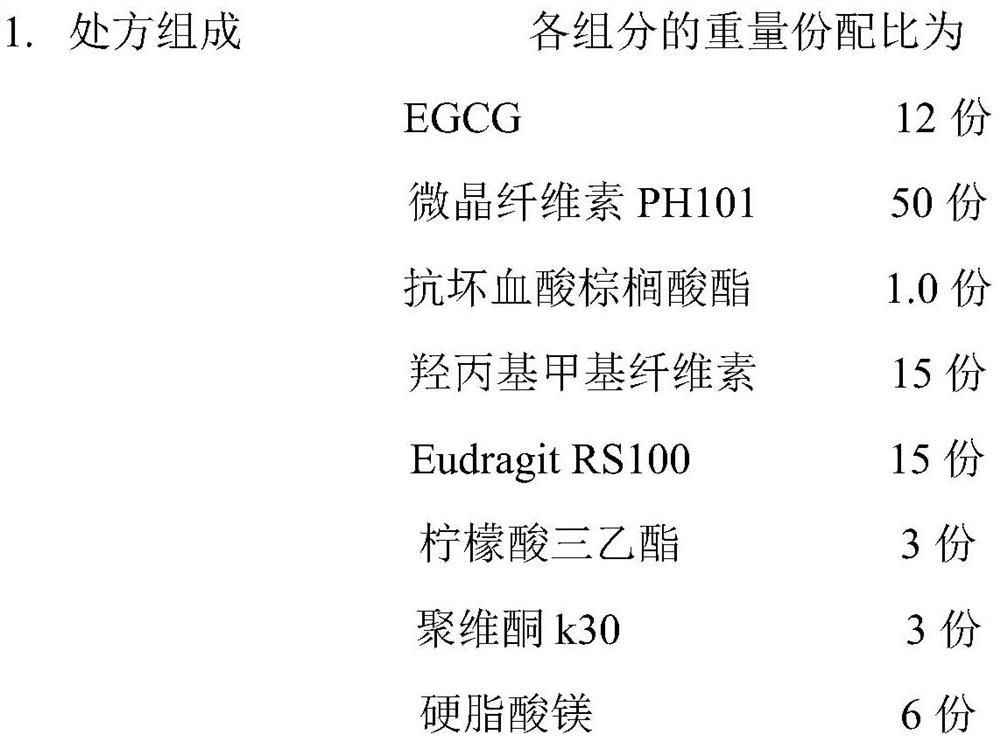
[0039] Pulverize epigallocatechin gallate with a pulverizer and pass through a 100-mesh sieve; weigh 12g of epigallocatechin gallate, 1g of ascorbyl palmitate, 15g of hydroxypropyl methylcellulose, and 50g of microcrystalline fiber Add Su PH101 to the wet granulator and mix evenly, then add an appropriate amount of aqueous solution to make a suitable soft material, stirring speed 400rpm, chopping speed 400rpm; extrude the soft material and spheronize to obtain wet skeleton pellets, extrusion speed 30rpm, spheronize The speed is 1000pm, the spheronization time is 5 minutes, and the wet pellet core is obtained; the wet pellet is dried in a fluidized bed for 10 minutes, and sieved to obtain a 20-40 mesh pellet core; weigh 15g Eudragit RS100, 3g triethyl citrate , 3g povidone k30, 6g magnesium stearate are dispersed in 90% ethanol, stir evenly, and preparation concentration is 5% coating liquid; Gained contains pill core coating, 60 ℃ of ...
Embodiment 2
[0041]
[0042] 1. Preparation method
[0043] Use a pulverizer to crush epigallocatechin gallate and pass through a 100-mesh sieve; weigh 10g of epigallocatechin gallate, 1g of ascorbyl palmitate, 20g of hydroxypropyl methylcellulose, and 80g of microcrystalline fiber Add Su PH101 to the wet granulator and mix evenly, then add an appropriate amount of aqueous solution to make a suitable soft material, stirring speed 400rpm, chopping speed 400rpm; extrude the soft material and spheronize to obtain wet skeleton pellets, extrusion speed 30rpm, spheronize The speed is 1000pm, the spheronization time is 5 minutes, and the wet pellet core is obtained; the wet pellet is dried in a fluidized bed for 10 minutes, and sieved to obtain a 20-40 mesh pellet core; weigh 20g Eudragit RS100, 4g triethyl citrate , 4g povidone k30, 4g magnesium stearate are dispersed in 90% ethanol, stir evenly, and preparation concentration is 5% coating solution; Gained contains pill core coating, 60 ℃ of ...
Embodiment 3
[0045]
[0046]
[0047] 1. Preparation method
[0048] Use a pulverizer to crush epigallocatechin gallate and pass through a 100-mesh sieve; weigh 20g of epigallocatechin gallate, 2g of ascorbyl palmitate, 20g of hydroxypropyl methylcellulose, and 60g of microcrystalline fiber Add Su PH101 to the wet granulator and mix evenly, then add an appropriate amount of aqueous solution to make a suitable soft material, stirring speed 400rpm, chopping speed 400rpm; extrude the soft material and spheronize to obtain wet skeleton pellets, extrusion speed 30rpm, spheronize The speed is 1000pm, the spheronization time is 5 minutes, and the wet pellet core is obtained; the wet pellet is dried in a fluidized bed for 10 minutes, and sieved to obtain a 20-40 mesh pellet core; weigh 20g Eudragit RS100, 4g triethyl citrate , 4g povidone k30, 10g magnesium stearate are dispersed in 90% ethanol, stir evenly, and preparation concentration is 5% coating liquid; Gained contains pill core coatin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com