Therapeutic factor xii antibody
A factor and antibody technology, applied in the direction of antibodies, antibody medical components, anticoagulation factor immunoglobulin, etc., can solve problems such as drug administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0382] Example 1: Materials and methods
[0383] This example describes the experimental procedures and materials used for the studies described in Example 2.
[0384] Generation of anti-FXII monoclonal antibody (MAb)
[0385] FXII-deficient mice were immunized with recombinant human FXII (Ivanov et al., Blood 2017; 129:1527–1537), and hybridomas were generated by standard methods. Clone 5C12 produced antibodies that bound FXII and FXIIa. Hybridoma cells were subcloned and expanded, and 5C12 was purified using standard procedures, characterized in vitro, and then used for in vivo experiments.
[0386] Characterization of 5C12
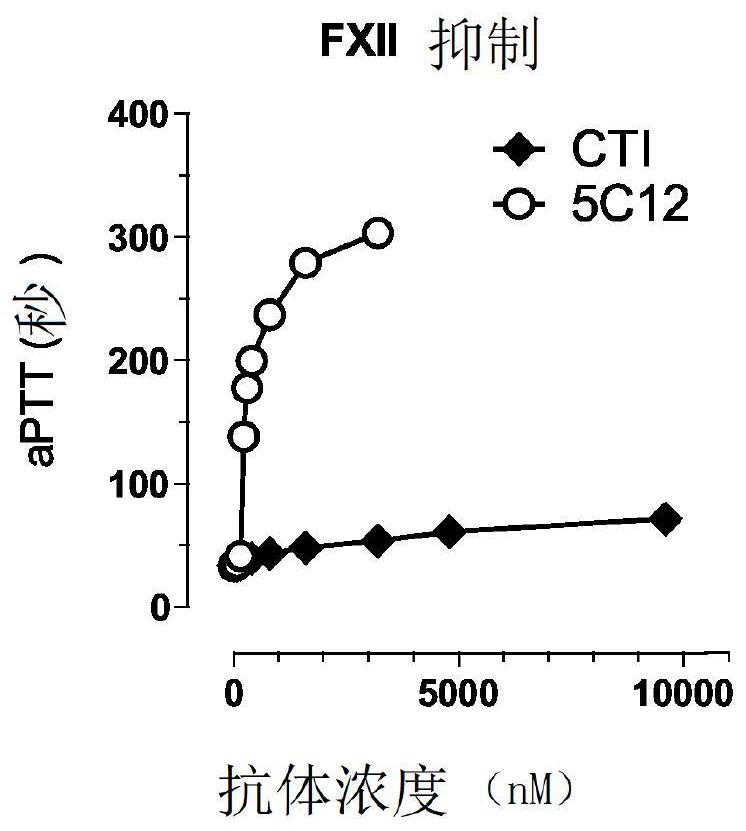
[0387] FXII (40 nM) (Haematologic Technologies, Inc., Essex Junction, VT) was incubated with 0-80 nM 5C12 (10 minutes) followed by 1 μg / ml dextran sulfate (20 minutes). Spectrozyme FXIIa (0.5 mM) (Sekisui Diagnostics GmbH, Pfungstadt, Germany) was then added to measure hydrolysis by activated FXII (FXIIa). Next, FXII (100nM) and 5C12 (0-200nM) were...
Embodiment 2
[0398] Example 2: Inhibition of Antibodies to Factor XII in Baboons Reduces Platelet Deposition from Blood in Extracorporeal Membrane Oxygenation
[0399] This example describes the characterization of FXII-specific monoclonal antibody 5C12 and its ability to act as an anticoagulant and reduce platelet aggregation. Furthermore, using a primate model, these studies show that FXII inhibition reduces platelet activation and deposition within membrane oxygenators in both heparinized and non-heparinized baboons.
[0400] 5C12 inhibits FXIIa
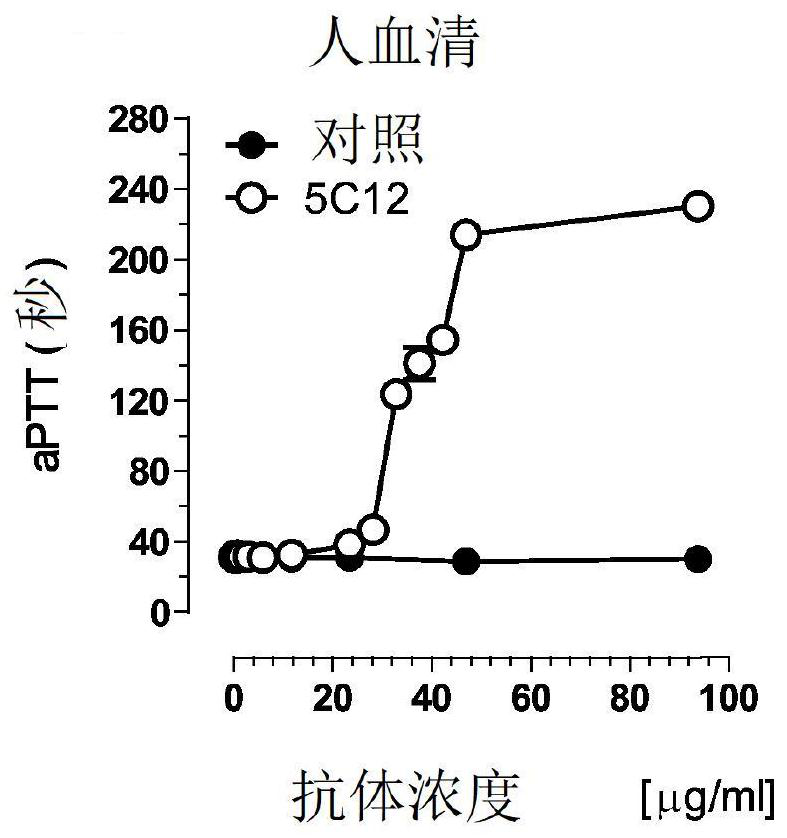
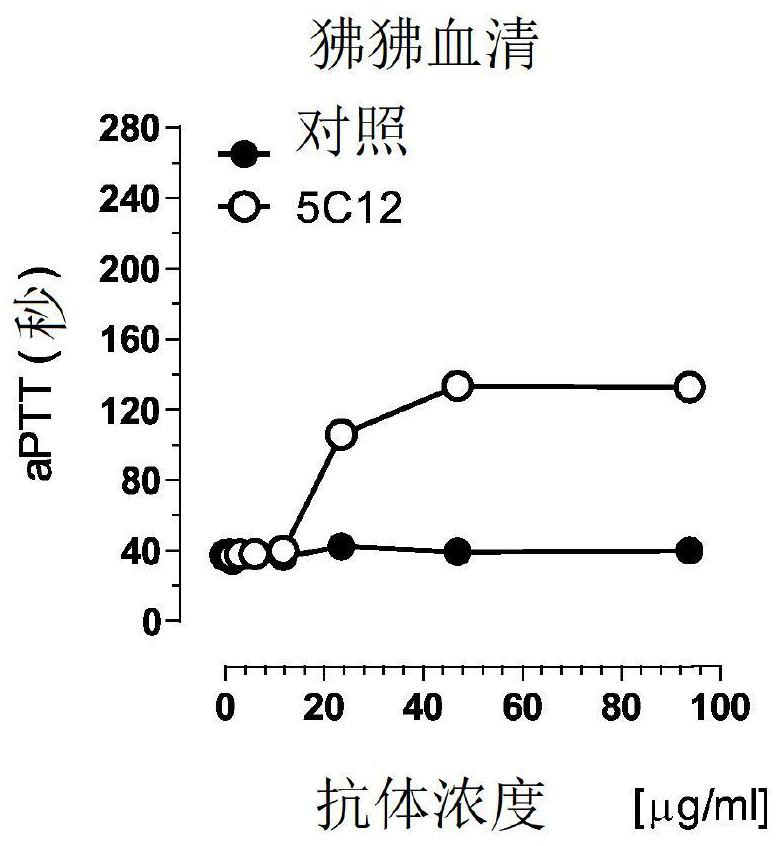
[0401] On Western blot, the 5C12 MAb recognized the α and β forms of FXII in humans and baboons and the African green monkey by binding to the protease domain, but not FXII from the other species tested ( Figures 3A-3C ), and prolonged the aPTT of human and baboon plasma mixed with FXII-depleted plasma ( Figure 2A-2B ), but did not extend the other tested species ( Figure 2C-2F ). Increasing the concentration of 5C12 prolongs the clotti...
Embodiment 3
[0416] Example 3: Evaluation of FXIIa inhibition using 5C12 (AB053) in a baboon model of lethal S. aureus exposure
[0417] This Example describes a study evaluating the effect of prophylactic 5C12 (hereinafter "AB053") in a baboon model of fatal systemic inflammatory response syndrome (SIRS). SIRS with or without intravascular coagulation and / or coagulopathy is sometimes observed in patients receiving bactericidal antibiotics to prevent or treat sepsis. Baboons were administered a "saturating" dose of AB053 (10 mg / kg) or no antibody intravenously immediately prior to a continuous 2-hour intravenous infusion of 30 billion heat-inactivated Staphylococcus aureus. Two additional doses of AB053 were administered after 8 hours (10 mg / kg) and 24 hours (5 mg / kg). Vital signs are monitored and blood samples are taken to measure organ function and markers of coagulation, inflammation, and organ damage. Untreated animals (n=3) developed severe SIRS and shock, with significant changes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com