A kind of 4-fluoro substituted arylamine compound and its synthetic method
A technology for aniline compounds and synthesis methods, which is applied to the preparation of amino compounds, the preparation of amino hydroxy compounds, chemical instruments and methods, etc., can solve the problems of expensive reagents, unsuitable for scale-up production, etc. Lipophilic effect
Active Publication Date: 2022-06-03
江苏笃行致远新材料科技有限公司
View PDF0 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
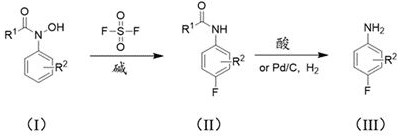
[0012] Literature Nature 2015,524,208-211 reported the use of aryl bromide, AgF and KF as a fluorine source under the catalysis of palladium for fluorination. This method is not suitable for scale-up due to the use of an air- and water-sensitive catalytic system, and the reagent is expensive. Production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
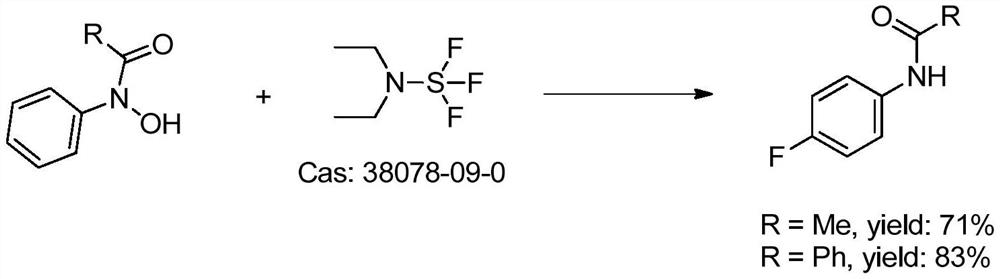
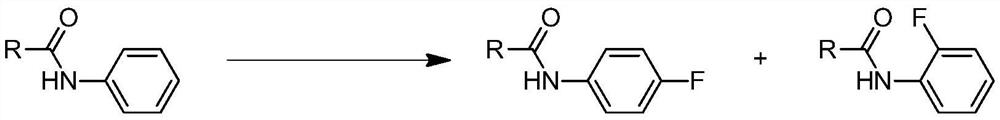
The invention discloses a method for synthesizing 4-fluorine-substituted arylamine compounds, which comprises the following steps: 1) taking acyl-protected phenylhydroxylamine as a substrate, and using sulfonyl fluoride as a fluorine source in a polar solvent, Generating 4-fluoro-substituted aniline compounds under basic conditions; 2) deprotecting groups under dilute acidic conditions or catalytic hydrogenation of Pd to obtain the 4-fluoro-substituted arylamine compounds. The synthetic 4-fluorine-substituted aniline compound of the present invention can be widely used in the preparation of fluorine-containing medicines, pesticides and dye intermediates because the introduction of fluorine atoms greatly increases its lipophilicity; meanwhile, the raw materials used in the present invention All are industrial products, cheap and easy to get, and are commercially available; the yield of 4-fluoroarylaniline prepared by the present invention is high, and a product with a purity of ≥99% can be obtained with a yield greater than 90%; the present invention is simple to operate , the low-cost process is very suitable for industrialization and can be widely used.
Description
A kind of 4-fluoro-substituted arylamine compound and synthetic method thereof technical field The invention belongs to the technical field of fine chemical industry, relate to the technical field of organic synthesis, particularly a kind of 4-fluorine substitution Arylamine compounds and synthetic methods thereof. Background technique Since fluorine is the most electronegative element, the introduction of fluorine atoms makes fluorine-containing organic compounds have a unique Physical properties, chemical properties and physiological activities make fluorine-containing organic compounds have chemical stability, surface activity and excellent Temperature resistance and other characteristics. Therefore, in many cutting-edge technologies and major industrial projects, as well as in pharmaceuticals, pesticides and other industries, fluorine-containing Compounds have been extensively and deeply researched and applied. Arylaniline substituted by p-fluorine is widely p...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C209/62C07C211/52C07C213/02C07C217/84C07C319/20C07C323/36C07C315/04C07C317/36
CPCC07C209/62C07C211/52C07C231/10C07C269/06C07C213/02C07C217/84C07C319/20C07C323/36C07C315/04C07C317/36C07C233/15C07C271/28C07C233/25C07C233/43C07C323/41C07C317/40Y02P20/55
Inventor 刘文娟刘智慧崔志鹏崔晓雷田瑞芳
Owner 江苏笃行致远新材料科技有限公司
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com