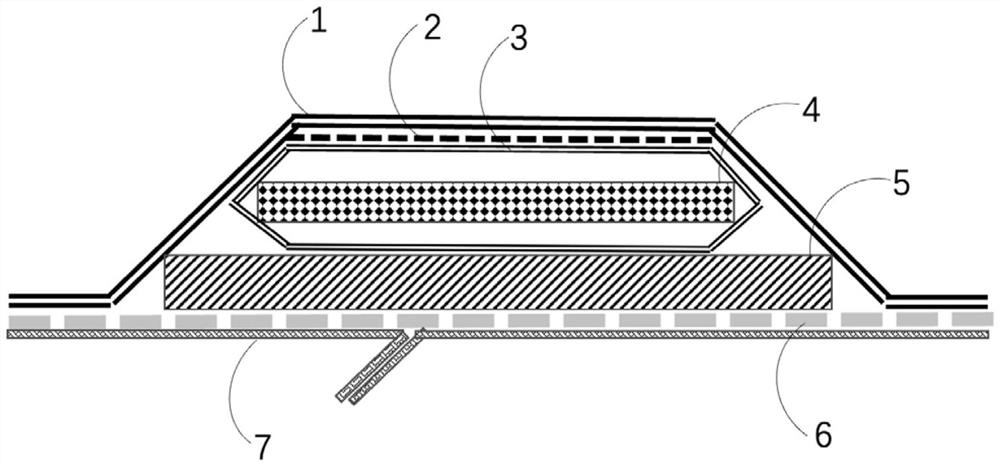
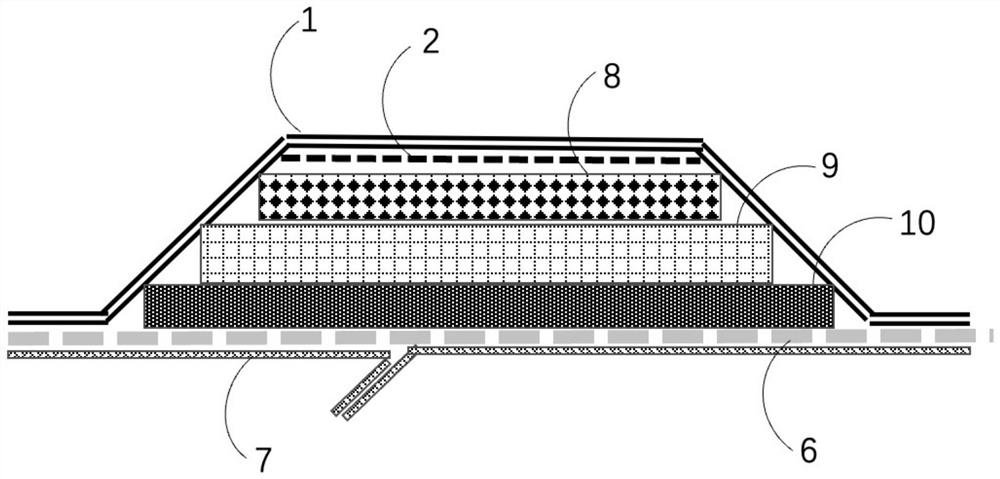
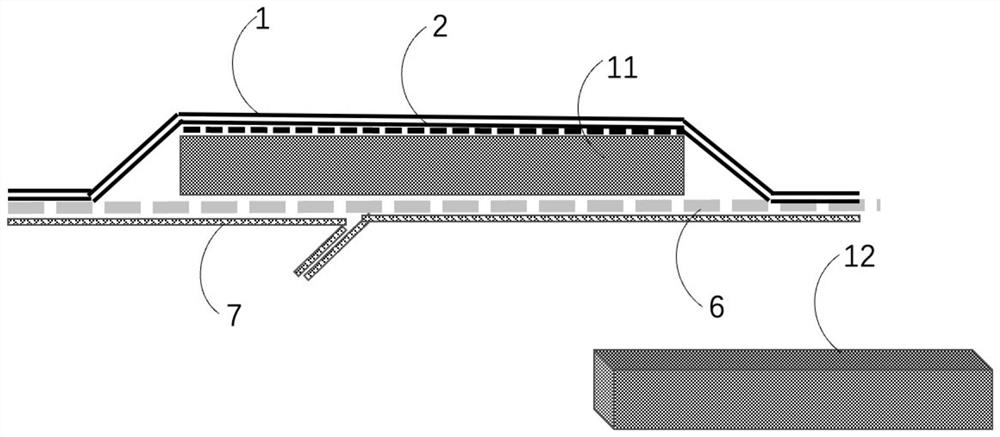
High-absorptivity wound dressing
A wound dressing, high absorbency technology, applied in the field of medical devices, can solve the problems of reduced absorption capacity, easy breakage, and aggravation of dressings, and achieves the effect of ensuring the absorption of exudate, ensuring fit and contact, and high adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Production process of alginate, pectin and carboxymethyl fiber composite antibacterial non-woven mat
[0146] 1) Preparation of spinning dope
[0147] In 200 kilograms of demineralized water, add 10 kilograms of sodium alginate (selected from Qingdao Mingyue Biomedical Materials Co., Ltd.) and 1 kilogram of carboxymethyl cellulose (CMC selected from Walocel of Dow Chemical in the United States) respectively. TM High-purity carboxymethyl cellulose) and 1 kg of high-methoxyl pectin (selected from Wuhan Dahua Weiye Medical Chemical Co., Ltd.), stirring and dissolving at room temperature for 24-48 hours, so that various substances are fully dissolved and defoamed;
[0148] 2) spinning forming
[0149] The above-mentioned spinning stock solution is extruded through the spinneret holes under pressure, solidified in the coagulation bath, and drawn 2-6 times in the drawing bath at 60-90°C;
[0150] 3) Drying and finishing
[0151] The fibers are washed with water, then dried...
Embodiment 2
[0156] Production process of alginate silver dressing
[0157] Produce composite gel fiber by the method for example 1 by the co-spinning of carboxymethyl cellulose fiber and calcium alginate fiber, calcium alginate fiber comes from domestic A company, and CMC fiber comes from Walocel of U.S. Dow Chemical TM High-purity carboxymethyl cellulose, the gel fiber contains 16% CMC and 84% calcium alginate, the thickness is about 2-6 denier, and the length is 4-5cm;
[0158] The non-gel fiber adopts the nylon 6.6 fiber (trade name ionic) that has been coated with 99.9% silver of Nobel Biomaterial company TM ), the thickness is about 100-150 denier, and the length is 4-5cm; the gel fibers and non-gel fibers are separated loosely and mixed according to 60% gel fibers and 40% non-gel fibers, and sent into the web The machine forms a carded fiber web, feeds the carded fiber web into a cross-lapper and folds it into a continuous 5-10cm thick cross-web, and then sends it to a needle punch...
Embodiment 3
[0159] Embodiment 3 Absorbency, wet and dry strength test method and test
[0160] 1. Test method for absorbency.
[0161] (1) Preparation of solution A: Take a 1L volumetric flask, dissolve 8.298 g of sodium chloride and 0.368 g of calcium chloride dihydrate with a small amount of deionized water and dilute to make 1 L. The solution contains 142mmol of sodium ions and 2.5mmol of calcium ions. The ion content of the solution is equivalent to that of human serum or wound exudate, and is used as a body fluid simulation fluid.
[0162] (2) Place the sample in a constant temperature and humidity oven for 24 hours, take it out and accurately cut it into 5x 5cm, and measure its actual size with a standard steel ruler;
[0163] (3) Place the empty Petri dish on the balance and tare it;
[0164] (4) Put a 5 x 5 cm dressing sample into a Petri dish and record the weight (W1) to 3 decimal places, remove the Petri dish and sample from the balance;
[0165] (5) Add solution A at 37°C t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com