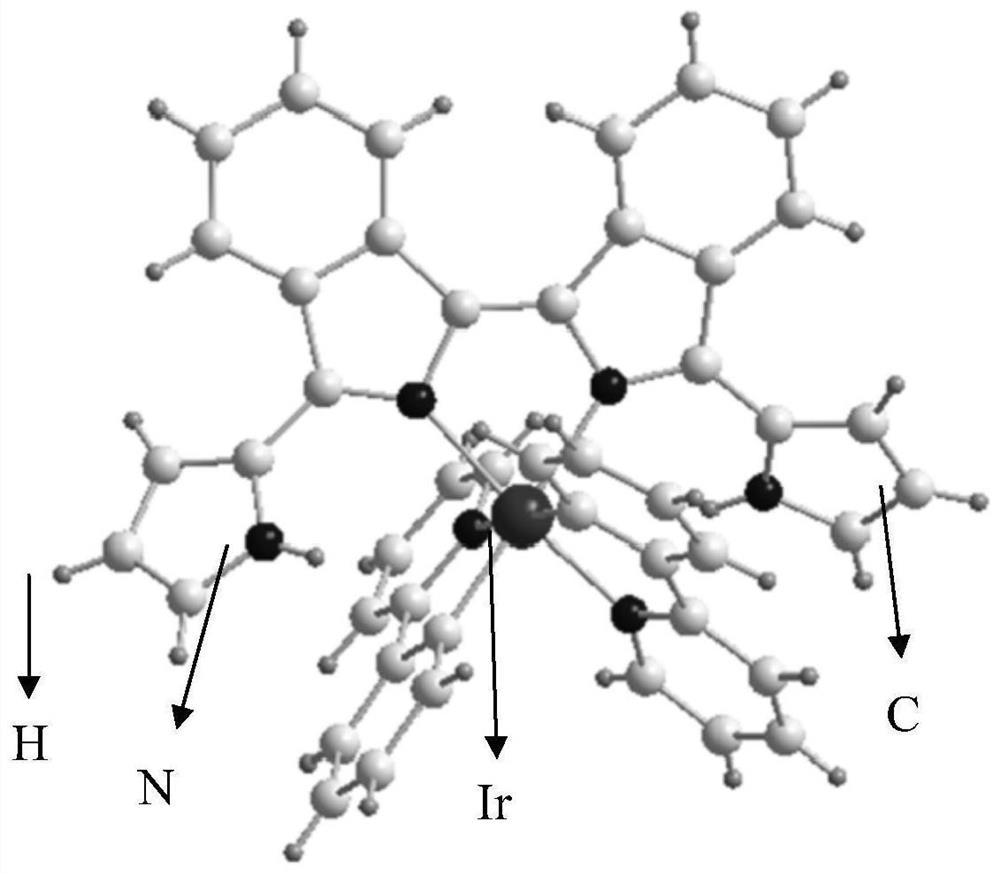
Diisoindole iridium (III) complex and preparation method thereof
A technology of indole iridium and complexes, which is applied in the field of bisisoindole iridium complexes and their preparation, can solve the problems of difficulty in synthesis, short absorption wavelength of complexes, inability to reach near infrared rays and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Correspondingly, the embodiment of the present invention also provides a preparation method of bisisoindole iridium (III) complex, comprising the following steps:
[0060] S10. Under the first protective gas atmosphere, obtain a mixed solution of isoindolinone compounds, pyrrole compounds and phosphorus oxychloride, and perform a first contact reaction to obtain a first product;
[0061] S20. Alkali treatment is performed on the first product to obtain bisisoindole ligand compound;
[0062] S30. Under the second protective gas atmosphere, after mixing the bisisoindole ligand compound, iridium (III) dimer and silver hexafluorophosphate with an organic solvent, perform a second contact reaction to separate and obtain bisisoindole Indole iridium(III) complexes.
[0063] The preparation method of the bis-isoindoline iridium (III) complex provided by the embodiment of the present invention is to carry out the first contact reaction of the isoindolinone compound and the pyrr...
Embodiment 1
[0078] A kind of synthesis of bisisoindole iridium (III) complex Ir-1:
[0079]
[0080] Under nitrogen protection, dissolve isoindolinone (100mg, 0.75mmol) and pyrrole (62μL, 0.90mmol) in anhydrous chlorobenzene (20mL), add phosphorus oxychloride (68μL, 0.75mmol), and mix The solution was heated to 110°C and stirred for 6h. TLC tracking spot plate, after the consumption of isoindolinone is complete, add sodium carbonate saturated solution containing potassium ferricyanide to the reaction system, continue stirring at room temperature for 2h, then transfer the reaction mixture to a separatory funnel, add dichloromethane Methane and water, extract, stand still, separate the organic phase, extract the corresponding aqueous phase with dichloromethane three times, and combine the organic layers. The organic phase was washed once with water, dried over anhydrous sodium sulfate, filtered, the solvent was concentrated in vacuo, and purified by silica gel column chromatography to o...
Embodiment 2
[0088] A kind of synthesis of bisisoindole iridium (III) complex Ir-2:
[0089]
[0090] Get the obtained bisisoindole ligand powder 54mg (0.15mmol), iridium (III) dimer Ir dimer2 (0.075mmol, 96mg) and silver hexafluorophosphate (0.15mmol, 38mg) in methanol and 1 , in a 1:1 system of 2-dichloroethane (total volume 20mL), stir at 80°C for 2h to obtain the target product, purify it with silica gel column chromatography, and then recrystallize it with dichloromethane and n-hexane to obtain Bisisoindole iridium(III) complex powder Ir-2, yield 47% (78mg).
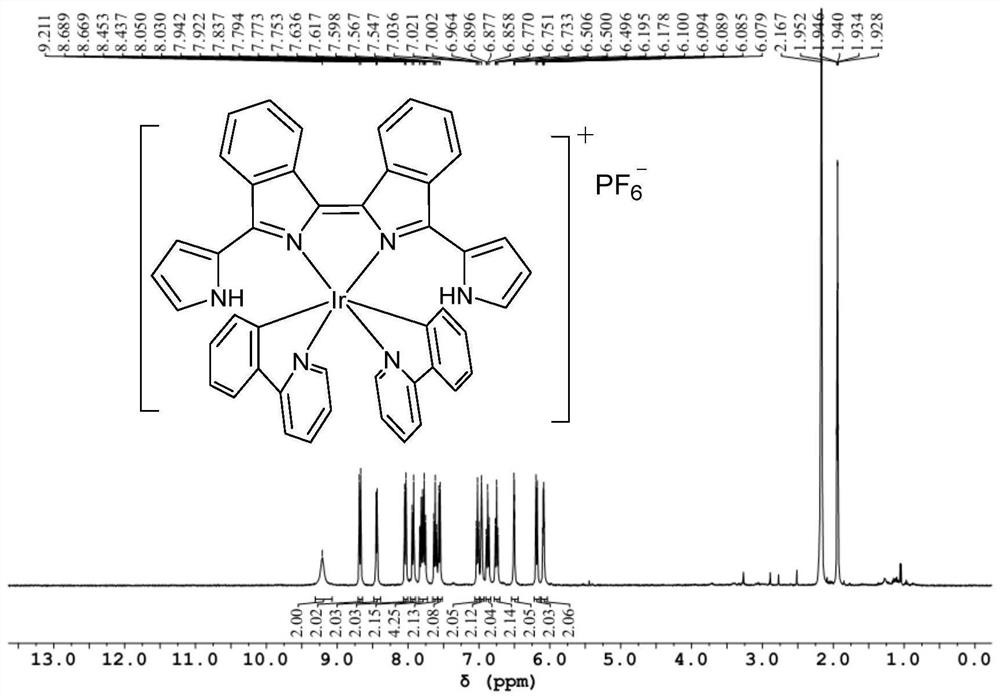
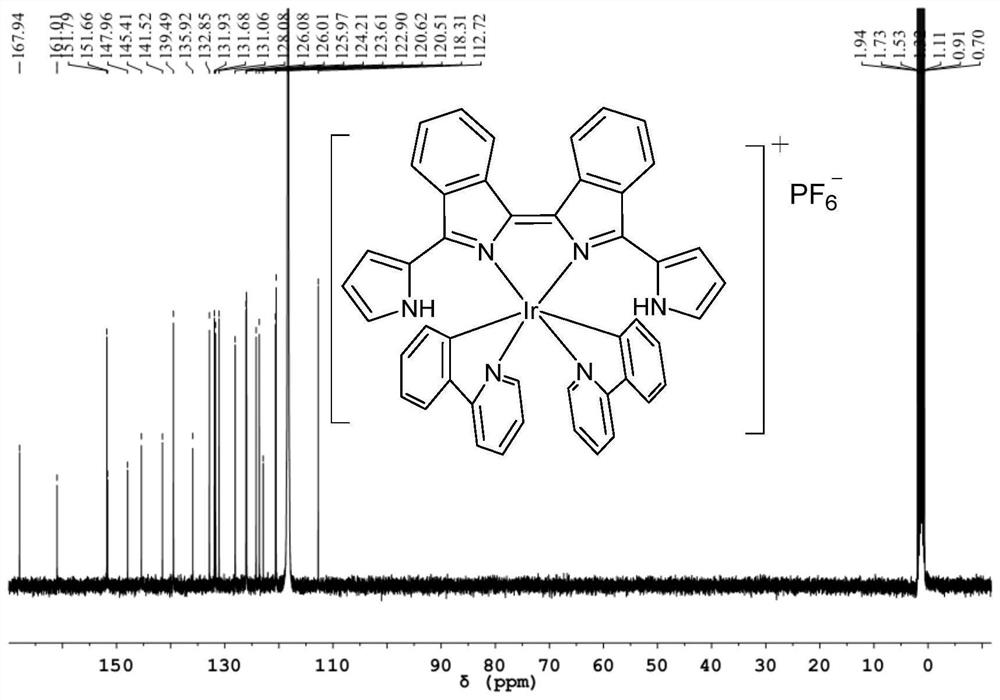
[0091] The NMR data and high-resolution mass spectrometry data of the bisisoindole iridium (III) complex Ir-2 are as follows:
[0092] 1 H NMR (400MHz, CD 3 CN)δ9.27(s,2H),8.67(dd,J=6.5,3.5Hz,2H),8.55(d,J=7.9Hz,2H),8.21(d,J=6.4Hz,2H),8.10 (d,J=8.1Hz,2H),7.95(d,J=7.9Hz,2H),7.86(dd,J=6.5,3.2Hz,2H),7.76–7.66(m,7H),7.52(t, J=7.7Hz, 2H), 7.35(d, J=6.4Hz, 2H), 7.01(t, J=7.6Hz, 2H), 6.89(s, 2H), 6.78(t, J=7.4Hz, 2H) ,6.48(s,2H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com