Method for preparing aromatic aldehyde
A technology for aromatic aldehydes and compounds, applied in the field of preparation of aromatic aldehydes, can solve the problems of high price of hexafluoroisopropanol, unfavorable industrial application, reduced efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6 and comparative example 1-3
[0054] Add respectively 0.5mmol nitric acid or nitrate (when being nitric acid, what add is the concentrated nitric acid aqueous solution of 66% mass fraction, wherein the molar weight of nitric acid molecule is 0.5mmol), 1mmol NHPI, 10mmol toluene and 20g 2,2,2-Trifluoroethanol (TFE), feed 1 atmosphere of pure oxygen (purity greater than 99%), replace the gas three times, in a pure oxygen atmosphere, heat the water bath to 50°C, start stirring, stirring mode For magnetic stirring, the stirring speed is 800 rev / min, start timing, after reacting for 24 hours, stop the reaction, after cooling to room temperature, sampling analysis (GC internal standard method analysis, internal standard is biphenyl), the specific results are shown in Table 1 shown.
[0055] Table 1
[0056]
[0057]
Embodiment 7-8 and comparative example 4-9
[0059] In the reactor of 50mL, add 0.02mol toluene, 10mol% (accounting for toluene mol ratio) NHPI, 5mol% (accounting for toluene mol ratio) Fe(NO 3 ) 3 9H 2 O, add 20g of different solvents to the reaction kettle, feed 1 atmosphere of pure oxygen (>99%) into the kettle, replace the gas three times, heat to 50°C in a pure oxygen atmosphere, start stirring, and the stirring speed is 800 rpm / minute, start timing, after reacting 24h, stop reaction, after being cooled to room temperature, sampling analysis (GC internal standard method analysis, internal standard is biphenyl), concrete result is shown in table 2.
[0060] Table 2
[0061]
Embodiment 9-15
[0063] In the reactor of 50mL, add the compound shown in 0.02mol formula (I), 10mol% (accounting for the compound molar ratio shown in formula (I)) NHPI, 5mol% (accounting for the compound molar ratio shown in formula (I)) Fe(NO 3 ) 3 9H 2 0, add 20g 2,2,2-trifluoroethanol in the reactor again as solvent, pass into the pure oxygen (>99%) of 1 atmospheric pressure in the still, replace gas three times, be heated to 50 under pure oxygen atmosphere ℃, start stirring, the stirring speed is 800 rev / min, start timing, after reacting for 24h, stop the reaction, after cooling to room temperature, sampling analysis (GC internal standard method analysis, internal standard is biphenyl), specific results refer to Table 3 shown.
[0064] table 3
[0065]
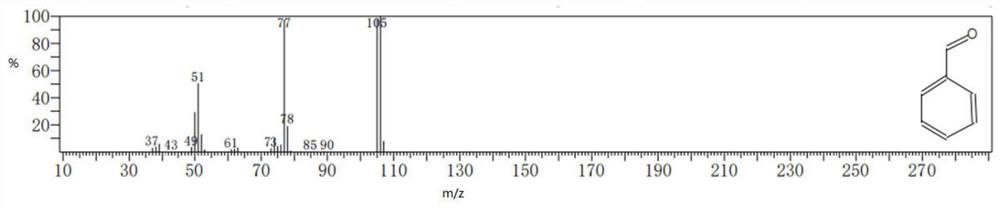
[0066] Measured: product in embodiment 9 Mass Spectrum See figure 1 shown.
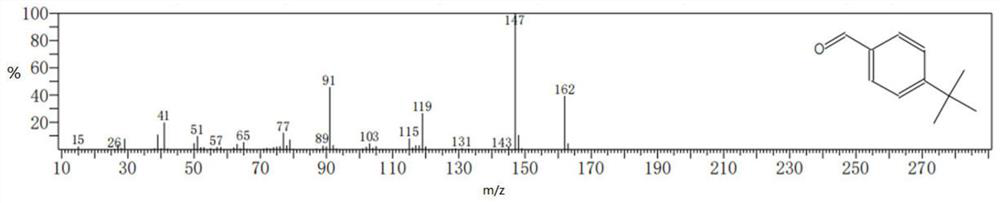
[0067] Measured: product in embodiment 10 Mass Spectrum See figure 2 shown.
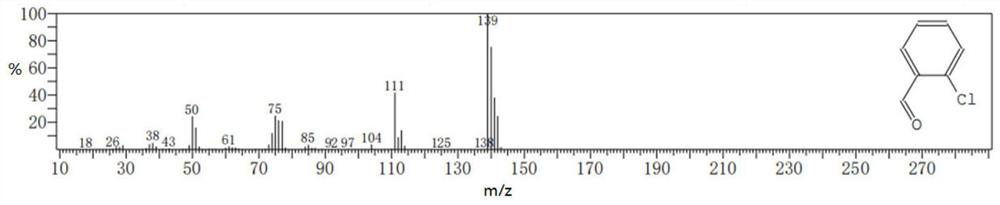
[0068]Measured: product in embodiment 11 Mass Spectrum See ima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com