Preparation method and application of thermal denaturation lysozyme with anti-influenza A virus activity
A technology of influenza A virus and heat denaturation, applied in the field of biomedicine, can solve the problems such as the research report on anti-influenza A virus of heat denatured lysozyme, etc., and achieve the effect of clinical transformation or market promotion with less toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: preparation of heat-denatured lysozyme
[0035] 1. Source of lysozyme
[0036] Natural egg white lysozyme was purchased from Suo Laibao Biotechnology Co., Ltd., item number: L8120.
[0037] 2. Preparation of denatured lysozyme
[0038] A, solution configuration: configuration mass volume ratio is 0.5%, 1%, 1.5%, 2%, 2.5%, 3%, 4%, 5% heat-denatured lysozyme, solvent is water;
[0039] B. pH adjustment: use NaOH, KOH, HCl or H 3 PO 4 Wait for the inorganic acid to adjust the pH value of the lysozyme solution to the desired value, usually 4 to 8. When the pH value is greater than 8, the egg white lysozyme will be difficult to dissolve in the solvent and precipitate;
[0040] C. Heat denaturation: after sterilizing the lysozyme solution through a 0.22 μm filter membrane, place it in a water bath or a metal bath, heat it at 75-100°C (preferably 90-100°C) for 2-3 hours, and immediately Take it out and place it on ice to cool down, and store it in a sealed c...
Embodiment 2
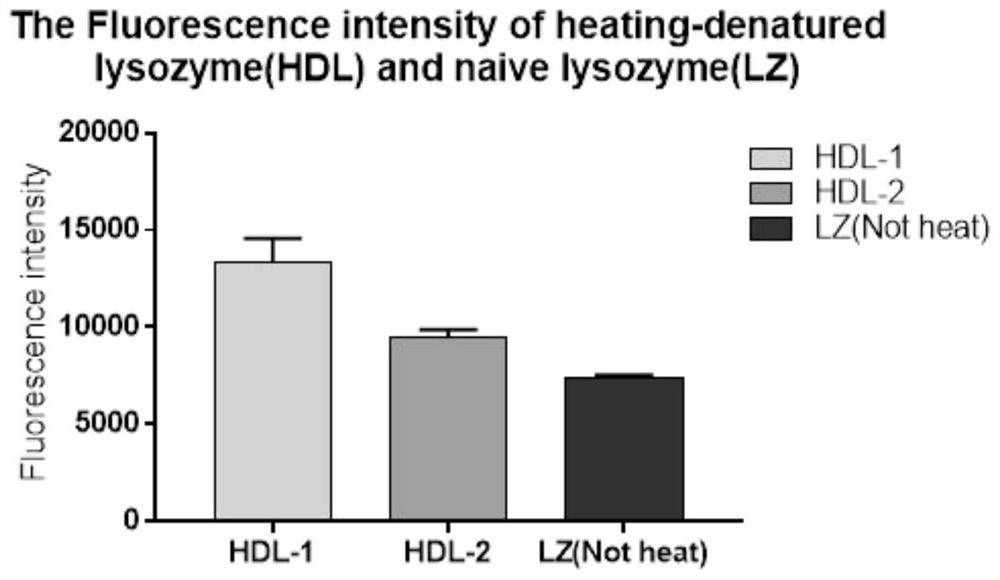
[0041] Embodiment 2: Hydrophobicity test of heat-denatured lysozyme (HDL)
[0042] 1. Materials
[0043] Orifice plate: 96-well non-removable microtiter plate (Corning, 3590)
[0044] Fluorescent agent: 8-aniline-1-naphthalenesulfonic acid (ANS, Solarbio, A9470)
[0045] Buffer: 0.1M PB (pH7.0)
[0046] 0.2M PB (pH7.0)
[0047] Microplate reader: Tecan infinite 200Pro
[0048] 2. Specific steps:
[0049] (1) Fluorescent agent ANS is dissolved in 0.1M PB, so that the final concentration is 8mM. ANS needs to be stored in the dark, and it is prepared and used immediately;
[0050] (2) The heat-denatured lysozyme was diluted with 0.2M PB to a concentration of 0.05%, that is, 0.5mg / mL;
[0051] (3) Add 10 μL of ANS solution to every 2 mL of diluted heat-denatured lysozyme solution, and use 0.2M PB solution as a control group, add 10 μL of ANS solution to the same 2 mL solution, mix well and react at room temperature for 30 minutes;
[0052] (4) Add 200uL of the mixed solutio...
Embodiment 3
[0055] Embodiment 3: in vitro anti-influenza virus plaque-loving experiment
[0056] 1. Materials:
[0057] DMEM basic medium (gibco, C11995500BT);
[0058] Fetal bovine serum (FBS gibco, 10270-106);
[0059] TPCK-trypsin (sigma, T1426-100MG);
[0060] PBS (Corning, 21-040-CV);
[0061] Penicillin / Streptomycin (p / s);
[0062] Orifice plate: 24-well plate (Nest, 702001);
[0063] Target cells: MDCK (Madin-Darby canine kidney) cell line;
[0064] Influenza virus: H1N1;
[0065] 2. Virus spotting test method:
[0066] (1) The genotype of the H1N1 virus is Influenza A / WSN / 33 virus (H1N1).
[0067] (2) 37°C, 5% CO 2 Cultivate MDCK cells, the medium is DMEM medium containing 10% fetal bovine serum (FBS), 1% penicillin / streptomycin (P / S), inoculated in the cell culture microwell plate one day before the experiment, it is required to be exactly plated A monolayer of cells in a well-filled plate.
[0068] (3) Dilute the virus infection titer used to 10 4~8 In between, mix t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| heat deflection temperature | aaaaa | aaaaa |
| heat deflection temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com