Synthesis method of nano titanium dioxide
A nano-titanium dioxide and synthesis method technology, applied in the direction of titanium dioxide, titanium oxide/hydroxide, nanotechnology, etc., can solve the problems of difficult control of the ratio, complicated post-processing procedures, and potential safety hazards, and achieve good catalytic activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] A kind of synthetic method of nano titanium dioxide, comprises the following steps:
[0053] The reaction raw materials are vaporized titanium tetrachloride, combustion-supporting gas and hydrogen mixed in the reactor, and the resulting mixture is ignited and enters the reaction furnace for combustion reaction. After the reaction, the obtained reaction product is input into the deacidification device and is flushed with the deacidification medium Carry out deacidification to obtain nano-titanium dioxide;
[0054] The temperature of the deacidification is 280~450°C, and the total time of the deacidification is 50~100min;
[0055] The temperature in the reaction furnace is 250-450°C.
[0056] In the process of preparing nano-titanium dioxide, the present invention finds that to realize the preparation of the rutile-anatase titanium dioxide mixed crystal form with a specific ratio of crystal form, especially to realize the preparation of the mixed crystal form of high-pro...
Embodiment 1
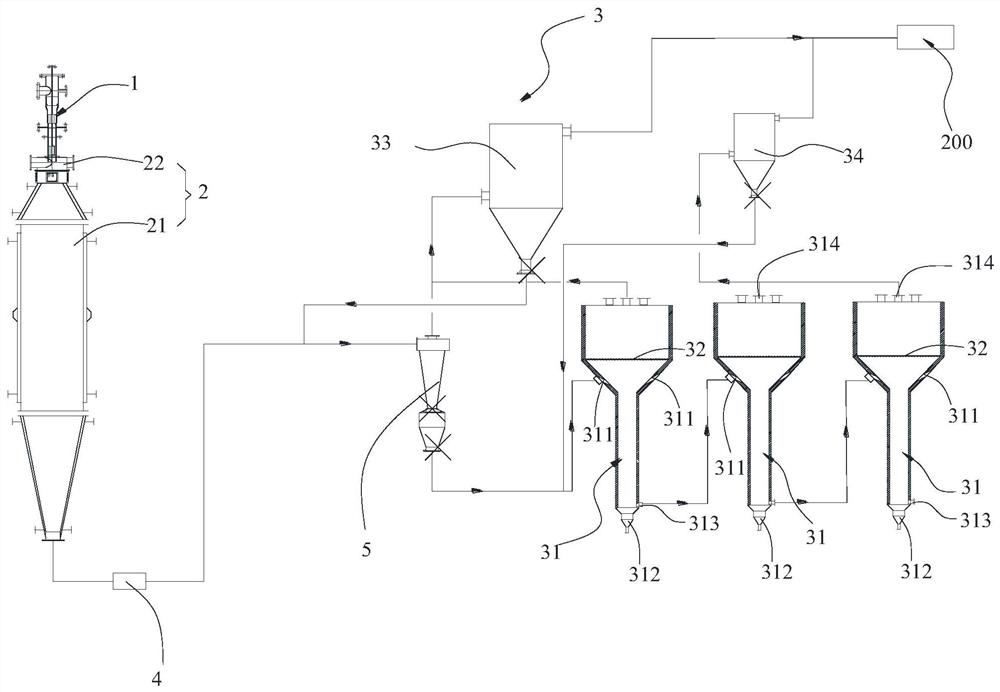
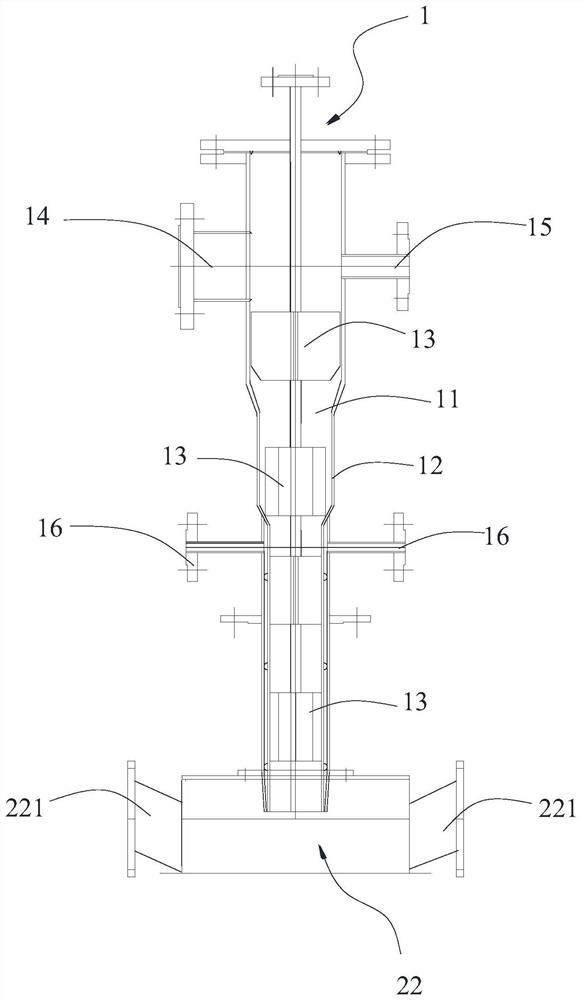
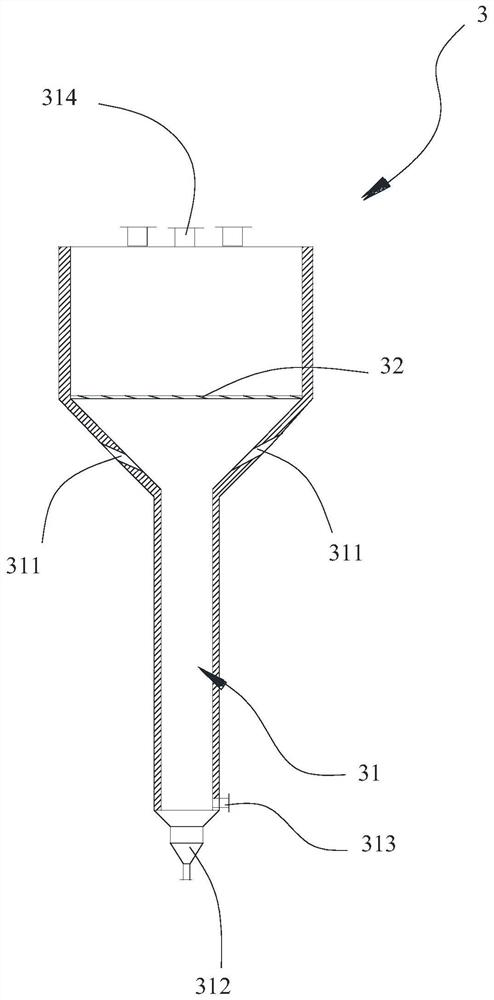
[0085] The device used in this example is figure 1 and figure 2 shown.
[0086] (1) Raw material TiCl 4 It flows into the vaporizer through the high level tank, the temperature of the vaporizer is controlled at 230°C, the outlet temperature of the vaporizer is greater than 150°C, the temperature of titanium tetrachloride is controlled at 160°C after vaporization, and the vaporization rate is 10kg / h;
[0087] (2) Vaporized TiCl 4 , enter the inner ring pipeline of the reactor 1 through the first feed port 14 together with the air after drying (control air carrier gas between 200-500 ℃) after the mass flowmeter metering; The reaction hydrogen is heated by the gas heater to Above 150°C, enter the inner ring pipe of the reactor from the second feed port 15. The flow rate of reaction air is 30kg / h, the flow rate of reaction hydrogen is 0.25kg / h; TiCl 4 1. After mixing air and hydrogen, the gas temperature is 200°C; a gas distribution plate is installed in the inner ring pipel...
Embodiment 2
[0091] The difference between this embodiment and embodiment 1 is:
[0092] In step (2), the flow rate of reaction hydrogen is 0.4kg / h, TiCl 4 1. After mixing air and hydrogen, the gas temperature is 220°C;
[0093] In step (3), the temperature in the reaction furnace 2 is 280° C., the auxiliary gas is air, the flow rate of the auxiliary gas is 5 kg / h, and the flow rate of the cooling gas is 0.2 kg / h.
[0094] Finally, the deacidified sample 2# was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com