Preparation of a quercetin combined hydrogen sulfide donor and its application in the treatment of diabetes and wound healing
A technology of quercetin and thione, applied in skin diseases, organic chemistry, metabolic diseases, etc., can solve the problems of no clinical significance, poor cell permeability, easy decomposition, etc., and achieves environmental protection of the solvent, simple operation steps, and raw materials. easy-to-get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
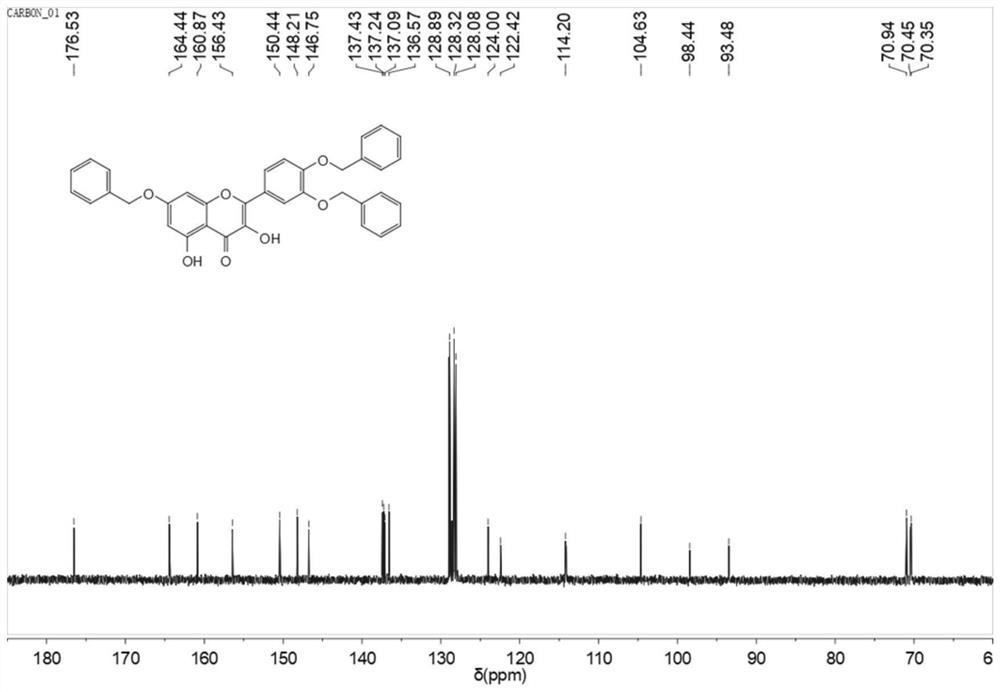
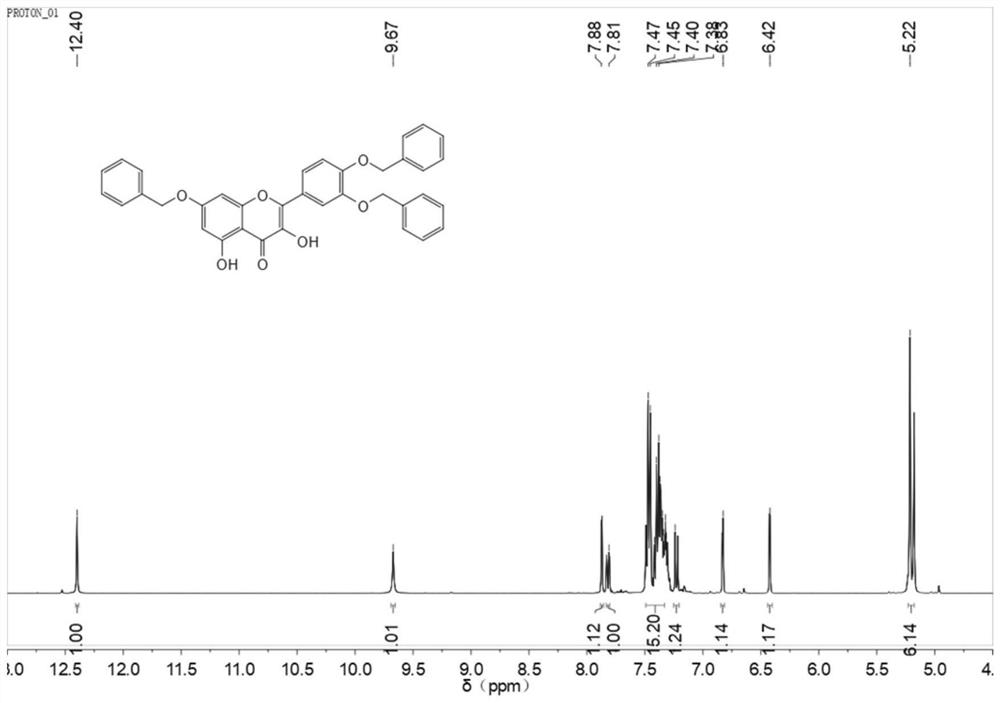
[0044] Embodiment 1, 3 ', 4 ', the synthesis of 7-O-tribenzyl quercetin
[0045]
[0046] Dissolve 8 mmol rutin in 40 ml DMF, then add anhydrous K 2 CO 3 28mmol, stirred under nitrogen for 0.5h, added dropwise 32mmol of benzyl bromide, reacted at 60°C for 3h, then adjusted the pH to 5 with glacial acetic acid (referring to glacial acetic acid aqueous solution with a concentration of 10% by volume), filtered, and dissolved the solid in 120ml Add 18ml of concentrated hydrochloric acid to ethanol, reflux for 2h, cool to room temperature, filter to obtain a yellow solid, filter and dry to obtain 3.5g of yellow crude product, the yield is 71.7%. 1 H NMR(400MHz,dmso)δ12.40(s,1H),9.67(s,1H),7.88(s,1H),7.81(s,1H),7.49-7.33(m,15H),7.24(s, 1H),6.83(s,1H),6.42(s,1H),5.22(s,6H). 13 C NMR(101MHz,dmso)δ176.53,164.44,160.87,156.43,150.44,148.21,146.75,137.43,137.24,137.09,136.57,128.89,128.32,128.08,124.00,122.42,114.20,104.63,98.44,93.48,70.94,70.45 ,70.35.m.p.190~192℃.
Embodiment 2
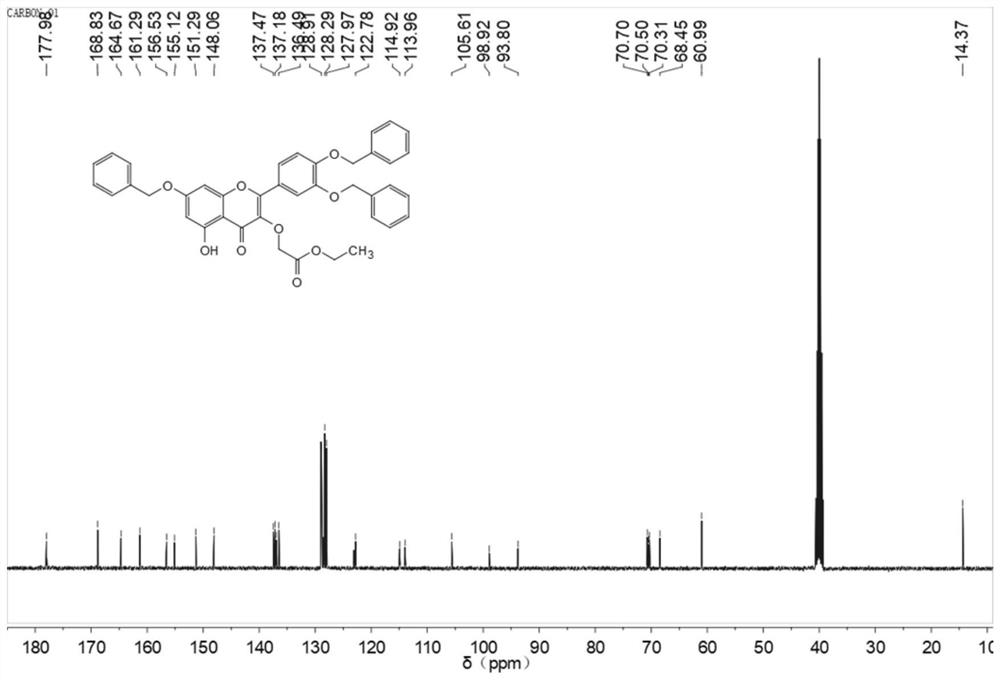
[0047] Embodiment two, 3', 4', the synthesis of 7-O-tribenzylquercetin-3-O-ethyl acetate
[0048]
[0049] Take 4mmol (2.3g) of 3',4',7-O-tribenzylquercetin prepared in Example 1 and dissolve in 60ml DMF, add anhydrous K 2 CO 3 5mmol, stirred at room temperature for 30min, then slowly added dropwise 15ml DMF solution containing 4.4mmol ethyl bromoacetate, reacted at room temperature for 2h, adjusted the pH to 6 with glacial acetic acid, extracted 3 times with ethyl acetate, collected the ethyl acetate layer, and used Wash with saturated brine, concentrate by rotary evaporation, and pass through the column (the eluent is acetone / petroleum ether with a volume ratio of 1:4) to obtain 0.5 g of light yellow filamentous solid with a yield of 21.7%. 1 H NMR (400MHz, dmso) δ12.47-12.31 (m, 1H), 7.88 (d, J=2.1Hz, 1H), 7.84-7.72 (m, 1H), 7.69-6.85 (m, 14H), 6.84- 6.59(m,1H),6.57-5.96(m,2H),5.54-5.21(m,4H),5.21-4.93(m,2H),4.79(d,J=16.9Hz,2H),4.21-3.97( m,2H),1.35-0.99(m,3H). 13 C...
Embodiment 3
[0050] Embodiment three, 3', 4', the synthesis of 7-O-tribenzylquercetin-3-O-acetic acid
[0051]
[0052] Take 4 mmol of 3',4',7-O-tribenzylquercetin-3-O-ethyl acetate prepared in Example 2 and 100 ml of distilled aqueous solution containing 200 mmol of NaOH, reflux for 3 hours, cool to room temperature, and adjust the pH to 2-3, extracted with ethyl acetate, washed the organic layer with water, concentrated by rotary evaporation, and dried to obtain 2.36 g of dark yellow powder solid with a yield of 90%. 13 C NMR(101MHz,dmso)δ178.11,170.36,164.63,161.30,156.49,154.91,151.25,148.09,137.48,137.18,136.97,136.48,128.95,128.27,127.99,122.81,114.85,113.89,105.60,98.86,93.69,70.66 ,70.49,70.32,68.33. 1 H NMR(400MHz,dmso)δ13.01(s,1H),12.45(s,1H),7.98(s,1H),7.75(s,1H),7.48-7.33(m,15H),7.18(s, 1H),6.80(s,1H),6.42(s,1H),5.21(s,6H),4.74(s,2H).m.p.164~165℃.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com