Method for synthesizing alpha, alpha-dideuterated alcohol and deuterated medicine through reduction deuteration of acyl fluoride compound
A synthesis method and compound technology, applied in the field of reductive deuteration of acyl fluoride compounds, can solve problems such as poor selectivity and poor atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
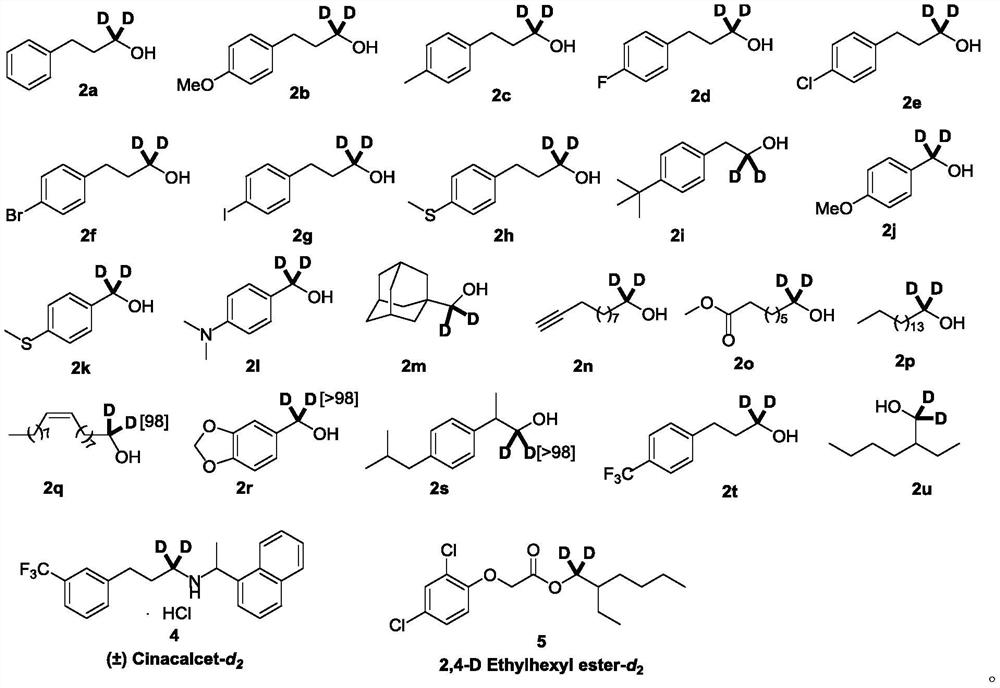
[0040] Into a 100mL single-necked round-bottom flask under the protection of argon, add samarium diiodide (SmI 2 ) in tetrahydrofuran (0.100mol / L) 50.0mL, compound 1a 150mg (1.00mmol), heavy water 601mg (30.0mmol). The reaction mixture was stirred at room temperature for 30.0 min, after which time the reaction was quenched by bubbling air. Ethyl acetate and 1.00M hydrochloric acid solution were added for extraction, the organic phase was dried, concentrated, and separated by column chromatography to obtain 131 mg of the target compound 2a with a yield of 95% and a deuteration rate of more than 98%.
[0041] The target product 2a obtained by the above synthesis method was detected by proton nuclear magnetic resonance spectrum and carbon spectrum, and the test results are as follows: 1 H NMR (500MHz, CDCl 3 )δ7.33–7.24(m,2H),7.21–7.16(m,3H),2.71(t,J=7.7Hz,2H),1.88(t,J=7.7Hz,2H),1.43(br,1H ); 13 C{ 1 H}NMR (126MHz, CDCl 3 )δ141.9, 128.5(×2), 125.9, 61.6(m), 34.1,...
Embodiment 2
[0043]
[0044] Into a 50mL single-necked round bottom flask under the protection of argon, add samarium diiodide (SmI 2 ) in tetrahydrofuran (0.100mol / L) 15.0mL, compound 1b 54.7mg (0.300mmol), heavy water 180mg (9.00mmol). The reaction mixture was stirred at room temperature for 30.0 min, after which time the reaction was quenched by bubbling air. Ethyl acetate and 1.00M hydrochloric acid solution were added for extraction, the organic phase was dried, concentrated, and separated by column chromatography to obtain 49.5 mg of the target compound 2b, with a yield of 98% and a deuterium substitution rate greater than 98%.
[0045]The target product 2b obtained by the above synthesis method was detected by proton nuclear magnetic resonance spectrum and carbon spectrum, and the test results are as follows: 1 H NMR (500MHz, CDCl 3 )δ7.11(m, 2H), 6.83(m, 2H), 3.78(s, 3H), 2.64(t, J=7.7Hz, 2H), 1.84(t, J=7.7Hz, 2H), 1.70( br,1H); 13 C{ 1 H}NMR (126MHz, CDCl 3 )δ157.8, 133.9,...
Embodiment 3
[0047]
[0048] Into a 50mL single-necked round bottom flask under the protection of argon, add samarium diiodide (SmI 2 ) in tetrahydrofuran (0.100mol / L) 15.0mL, compound 1c 49.9mg (0.300mmol), heavy water 180mg (9.00mmol). The reaction mixture was stirred at room temperature for 30.0 min, after which time the reaction was quenched by bubbling air. Ethyl acetate and 1.00M hydrochloric acid solution were added for extraction, the organic phase was dried, concentrated, and separated by column chromatography to obtain 44.8 mg of the target compound 2c with a yield of 98% and a deuterium substitution rate of 98%.
[0049] The target product 2c obtained by the above synthesis method was detected by proton nuclear magnetic resonance spectrum and carbon spectrum, and the test results were as follows: 1 H NMR (500MHz, CDCl 3 )δ7.11–7.07 (m, 4H), 2.66 (t, J = 7.8Hz, 2H), 2.31 (s, 3H), 1.85 (t, J = 7.8Hz, 2H), 1.65 (br, 1H); 13 C{ 1 H}NMR (126MHz, CDCl 3 )δ138.8, 135.4, 129.1, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com