Thin-layer chromatography identification method of rhizoma corydalis stomach'an capsule
A technology of thin-layer chromatography and chromatography, applied in the field of drug analysis, to achieve the effects of clear judgment of results, elimination of interference, and control of drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] (1) Preparation of the test solution:
[0063] Take 2 g of Yanhu Wei'an Capsules, add 20 ml of solvent for ultrasonic or reflux treatment for 30 minutes, filter, concentrate the filtrate to 2 ml, filter with a 0.45 μm microporous membrane, and use it as the test solution;
[0064] (2) Thin-layer chromatographic identification of chicken saigenin:
[0065] Take 1.0 mg of gallinaceous savine reference substance, dissolve it in 1 mL of methanol, filter it with a 0.45 μm microporous membrane, and use it as the reference substance solution;
[0066] Draw 2 μL each of the test solution and the reference solution, respectively, and place them on the same thin-layer chromatography plate, use chloroform-methanol-glacial acetic acid-water solution as the developing agent to develop upward, take it out, and dry it in the air; spray the thin-layer chromatography with 10% sulfuric acid ethanol solution, heated at 105°C until the color of the spots is clear; the chromatogram of the ...
Embodiment 1
[0097] Exploration of Qualitative Conditions of Thin Layers
[0098] 1. Choice of extraction solvent
[0099] ①Weigh 500g of each raw medicinal material. Then the 500g raw medicinal material is divided into two parts of 400g and 100g. The 400g part was heated and extracted with 10 times the mass of water, boiled for 30 minutes, then filtered and concentrated to 100ml; the 100g part was ultrasonically extracted by adding 5 times the mass of methanol for 30 minutes, filtered and concentrated to 100ml.
[0100]② Take 8g of Yanhu Wei’an Capsules, 6 parts in total, 3 parts were ultrasonicated with 10 times the mass of 75% ethanol, methanol and water for 30 minutes; the remaining 3 parts were heated in a water bath with 10 times the mass of 75% ethanol, methanol and water respectively Reflux for 30 minutes, and filter to obtain the filtrate.
[0101] ③ According to the mass ratio in the formula of Yanhu Wei'an Capsules, take the original medicinal materials to make capsules, a to...
Embodiment 2
[0116] TLC qualitative identification
[0117] Reference substances: all were provided by Chengdu Aifa Biotechnology Co., Ltd. (Chengdu, China), with a purity greater than 98%.
[0118] TLC plate: silica gel G plate of Qingdao Ocean Chemical Co., Ltd.
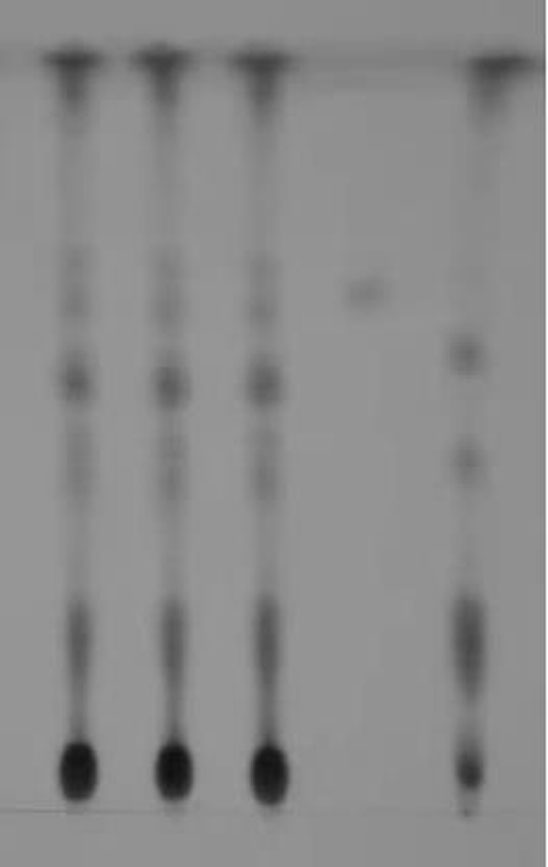
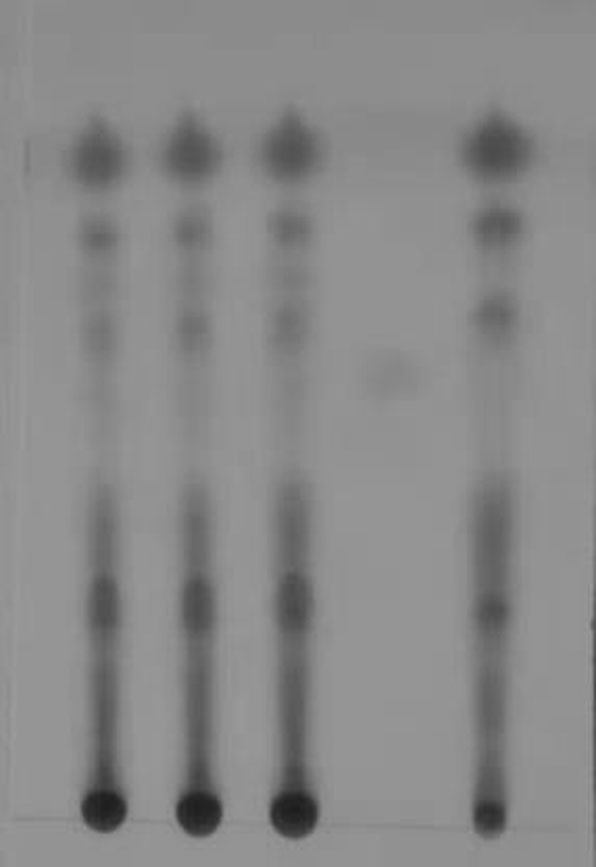
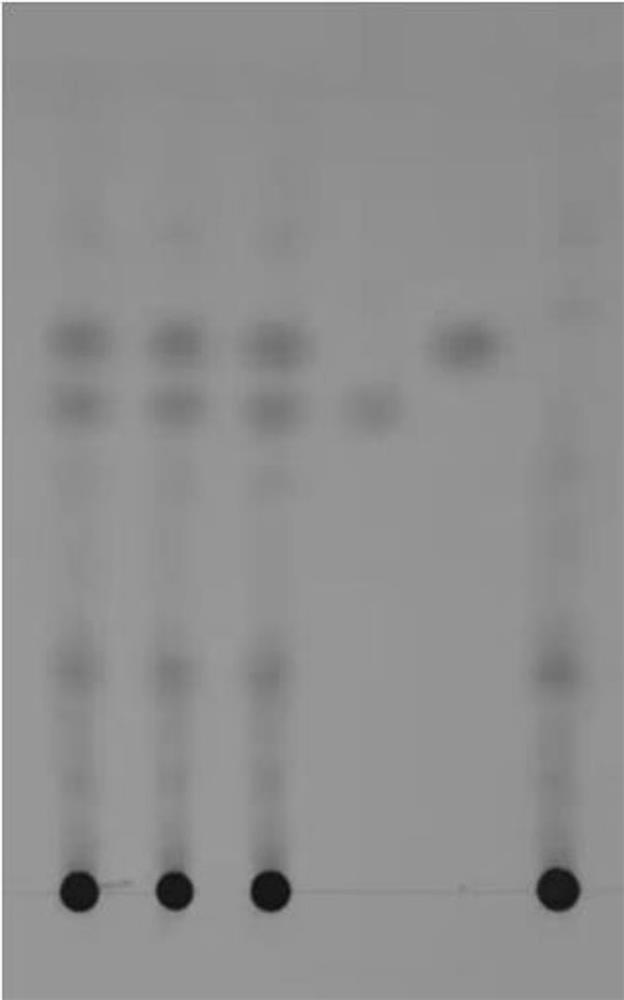
[0119] TLC identification: the first 3 points in the thin-layer plate are three batches of capsule test solution, the batch numbers are: 20190701LSH01, 20200701 LSH95, 20201101 LSH31, the fourth point is the reference substance, and the fifth point is the capsule negative solution.
[0120] 1. Method for TLC identification and determination of chicken savine glycosides
[0121] 1.1 Preparation of the test solution
[0122] Make capsules according to the prescription and process, take 2g of capsules, add 20ml of methanol, ultrasonically treat for 30 minutes, filter, concentrate the filtrate to 2ml, filter with a 0.45μm microporous membrane, and use it as the test solution;
[0123] 1.2 Preparation of reference medicinal solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com