Method for establishing fingerprint spectrum of flavonoid components in ginkgo leaf extraction intermediate or preparation thereof and fingerprint spectrum established by method
A technology of fingerprint spectrum and establishment method, which is applied in the field of fingerprint spectrum, the establishment of the fingerprint spectrum of flavonoids in Ginkgo biloba extract intermediates or their preparations, can solve the problems of not truly and comprehensively reflecting the flavonoids, and achieves easy identification, Monitor quality, peak shape is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0258] Example 1 Choice of detection wavelength
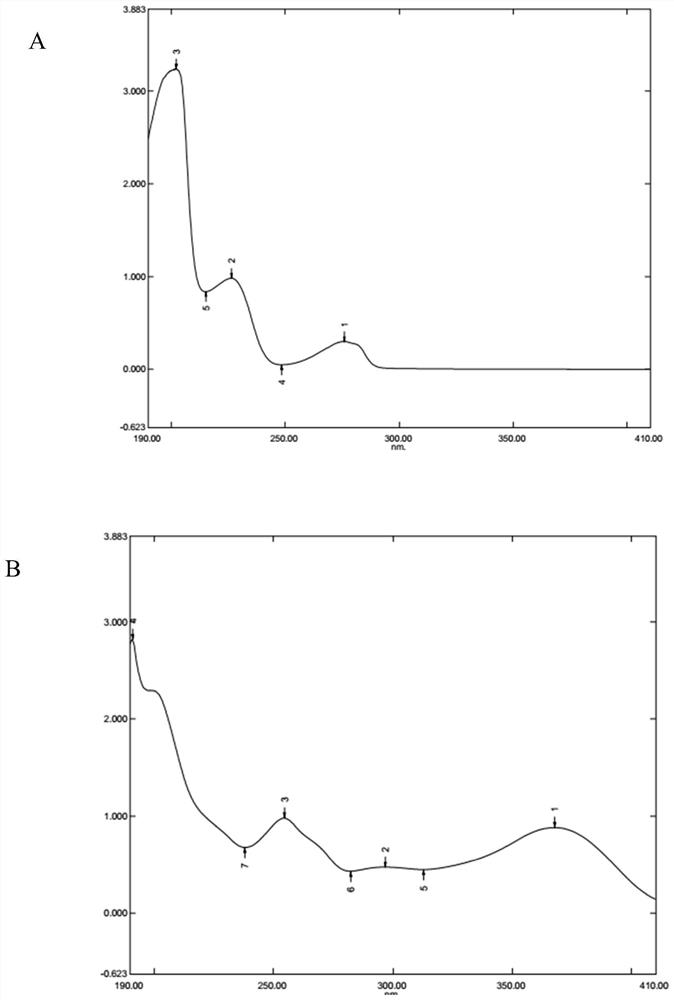
[0259] UV full-wavelength scanning of representative pinoresinol diglucoside and flavonoids representative of lignans in the intermediate of Ginkgo biloba extract, see figure 1 .
[0260]Preparation of quercetin reference substance solution: Accurately weigh an appropriate amount of quercetin reference substance, dilute it with 50% (v / v) methanol aqueous solution to a solution containing 0.012 mg per 1 ml, shake well, and obtain.
[0261] Preparation of pinoresinol diglucoside reference substance solution: Accurately weigh an appropriate amount of pinoresinol diglucoside reference substance, dilute with 50% (v / v) methanol aqueous solution to a solution containing 44.6 μg per 1 ml, shake well, and obtain.
[0262] From figure 1 It can be seen that the flavonoids present a characteristic maximum absorption wavelength near 360nm (see figure 1 B below); lignans do not absorb at 360nm but absorb at 265nm (see figure 1 A) above...
Embodiment 2
[0263] Example 2 Column Selection
[0264] Preparation of the test sample: Accurately measure 2ml of Shuxuening injection, put it in a 10ml measuring bottle, dilute to the mark with 50% (v / v) aqueous methanol solution, shake well, filter, and take the subsequent filtrate to obtain final product.
[0265] Chromatographic conditions: Acetonitrile was used as phase A, 0.1% (v / v) formic acid aqueous solution was used as phase B, and the gradient elution program was shown in Table 1.
[0266] Table 1 Gradient elution program (volume percentage)
[0267] time (min) Acetonitrile (%) 0.1% formic acid aqueous solution (%) 0 18 82 12 21 79 15 25 75 25 35 65
[0268] Flow rate 0.25ml / min;
[0269] Column temperature: 35°C;
[0270] Detection wavelength: 360nm;
[0271] Injection volume: 1 μl.
[0272] (1) Column 1
[0273] Model: Agilent ZORBAX SB-C18 (3.0×150 mm, 1.8 μm).
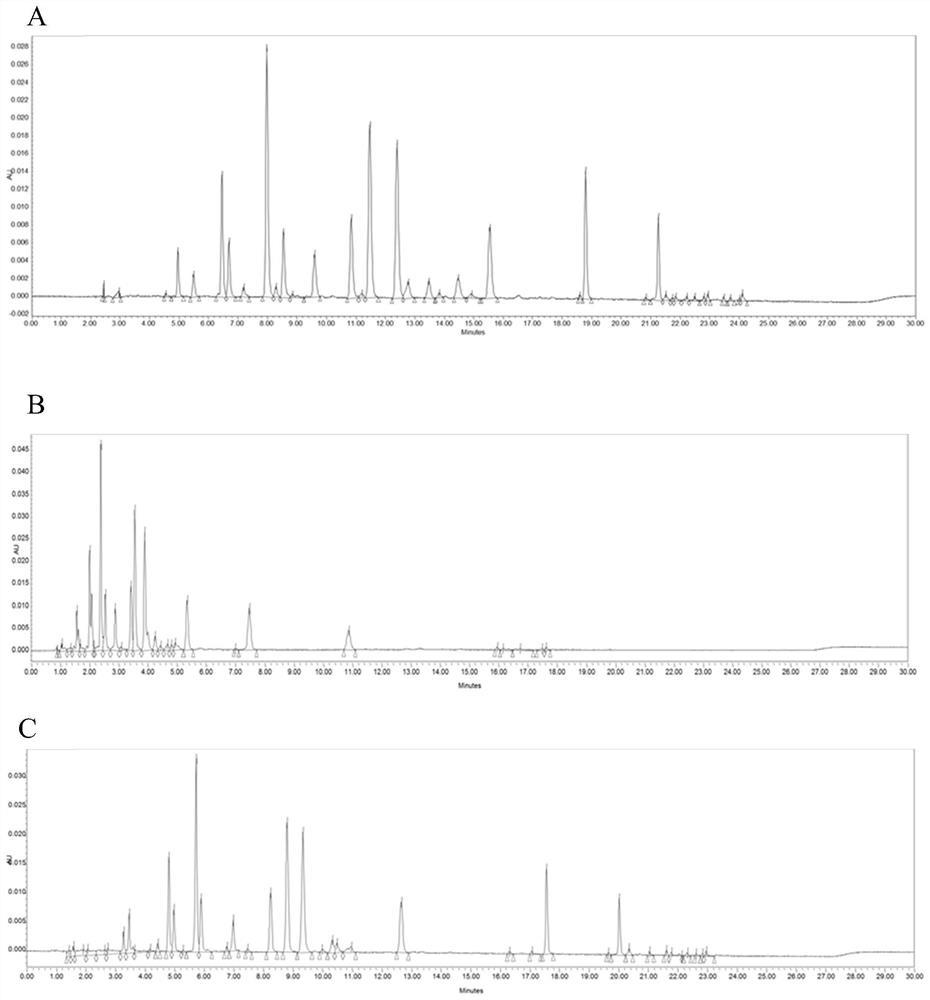
[0274] Chromatogram see figure 2 A.
[0275] (2) Column 2
...
Embodiment 3
[0283] Example 3 Choice of mobile phase
[0284] The method for preparing need testing solution according to embodiment 2, chromatographic column is Agilent ZORBAX SB-C18 (3.0 * 150mm, 1.8 μ m), other chromatographic conditions are the same as embodiment 2 except mobile phase.
[0285] (1) Mobile phase: acetonitrile-water
[0286] The gradient elution program is shown in Table 2.
[0287] Table 2 Acetonitrile-water gradient elution program (volume percentage)
[0288] time (min) Acetonitrile (%) water(%) 0 18 82 12 21 79 15 25 75 25 35 65
[0289] Chromatogram see image 3 A above.
[0290] (2) mobile phase methanol-water
[0291] Using methanol-water mobile phase, the system pressure is high, which is close to the maximum pressure of the chromatographic column, so it is not suitable to use methanol-water as the mobile phase.
[0292] (3) mobile phase acetonitrile-0.1% formic acid aqueous solution
[0293] The gradient elution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com