Reference product for detecting pathogenic microorganisms of bloodstream infection and preparation method thereof
A technology for detection of pathogenic microorganisms and infections, which is applied in the field of reference products for the detection of pathogenic microorganisms in bloodstream infections and its preparation, can solve problems such as the inability to evaluate sequencing platforms and detection methods, lack of reference materials for pathogenic microorganisms in bloodstream infections, etc., and achieve stable sources Reliable, avoiding experimental errors, and accurate value setting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
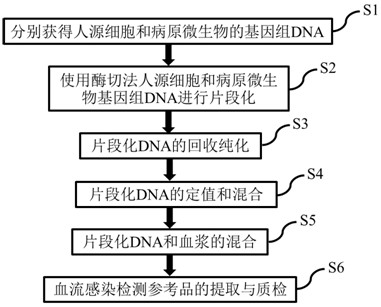
[0042] The invention discloses a method for preparing a reference product for bloodstream infection pathogenic microorganism detection that covers various pathogenic bacteria and is close to human natural plasma. The specific steps are as follows, and the process is as follows: figure 1 Shown:
[0043] S1: Extract the genomic DNA of cultured human cells and pathogenic microorganisms respectively; if the pathogenic microorganisms cannot be cultured, the target fragment can be synthesized artificially, and then a 200bp pathogenic microorganism-specific fragment can be obtained by PCR amplification.
[0044] S2: Fragmentation of genomic DNA of human cells and pathogenic microorganisms by restriction enzyme digestion.
[0045] The fragmentation enzyme is Fragmentase from NEB Company, and other commercial enzymes with equivalent effects can also be used, such as Yisheng OnePot, etc.;
[0046] S3: Recovery and purification of fragmented DNA.
[0047]The purification magnetic beads...
Embodiment 1
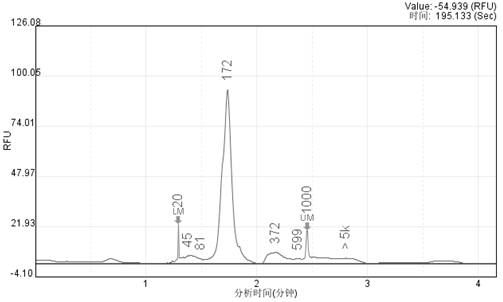
[0054] Example 1 Preparation of fragmented pathogenic microorganism genome DNA reference product
[0055] (1) Genomic DNA fragmentation of human cells and pathogenic microorganisms:
[0056] Three common pathogens of bloodstream infection were selected: Epstein-Barr virus, Pseudomonas aeruginosa, and Candida parapsilosis, representing viruses, bacteria, and fungi, respectively, and Hela cells were used as human cells. The genomic DNA of Hela cells, Epstein-Barr virus, Pseudomonas aeruginosa, and Candida parapsilosis were respectively digested by enzyme digestion, and the enzyme digestion system was prepared according to the following table (20 μl):
[0057]
[0058] Enzyme digestion was carried out at 37°C, and the amount of DNA input and digestion time were optimized as shown in the table below:
[0059]
[0060] After the fragmentation is completed, use XP magnetic beads and 80% ethanol to purify once, and the eluted DNA after purification is used
[0061] Kit dete...
Embodiment 2
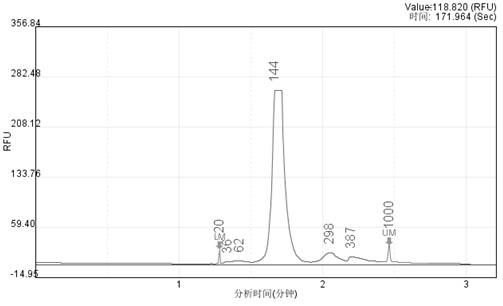
[0070] Embodiment 2 Fragmented Genome and PCR Amplified Fragment Mixed Reference Substance Preparation
[0071] Because some pathogenic microorganisms are difficult to obtain pure culture for genomic DNA preparation due to pathogenicity or non-growth in vitro, such as mycoplasma, tuberculosis, rickettsia, mold, etc., so artificial synthesis and PCR are required DNA is obtained by amplification.
[0072] (1) PCR amplified fragments obtained:
[0073] In this example, Mycobacterium tuberculosis, Ureaplasma urealyticum, and Aspergillus terreus were used as representatives. Two species-specific sequences of each pathogen were obtained by bioinformatics means, and then two target fragments were amplified by pathogen-specific primers. The target fragments were both At about 200bp, the pathogen-specific primer sequences are shown in the table below:
[0074]
[0075] Target fragment PCR amplification system and procedures are as follows:
[0076]
[0077] After PCR, the prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com