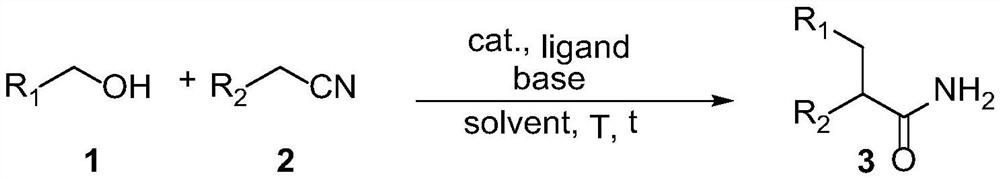
A kind of method of synthesizing aromatic acetamide
An aromatic acetamide and compound technology, which is used in the fields of pesticides, organic chemicals, and medicine to avoid the use of alkylating reagents, reduce amide synthesis steps, and save costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
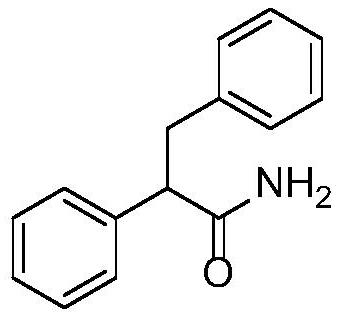
[0026] Example 1: Synthesis of 2,3-diphenylpropanamide
[0027]
[0028] Benzyl alcohol (0.216 g, 2.0 mmol) and phenylacetonitrile (0.117 g, 1.0 mmol) were dissolved in 1.0 mL of tert-amyl alcohol and mixed with 7.9 mg (1.0 mol%) of [Cp*IrCl 2 ] 2 , 3.6 mg (2.0 mol %) of 1,10-phenanthroline, and 22.4 mg (20 mol %) of potassium tert-butoxide were added to a pressure-resistant tube, and after nitrogen replacement, the reaction was carried out at 140° C. for 6 h. The obtained reaction solution was cooled to room temperature, extracted with water and ethyl acetate, the organic phase was separated, dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by rapid preparative liquid chromatography (volume ratio of ethyl acetate: petroleum ether was 30:100). The eluate containing the target compound was collected, and 0.203 g of the compound represented by formula 3 was obtained by rotary evaporation under reduced pressure, with a yield of 90% and a liquid pha...
Embodiment 2
[0029] Example 2: Synthesis of 2,3-diphenylpropanamide
[0030] Benzyl alcohol (0.108 g, 2.0 mmol) and phenylacetonitrile (0.117 g, 1.0 mmol) were dissolved in 0.5 mL of tetrahydrofuran and mixed with 1.4 mg (0.2 mol%) of [Ir(cod)Cl 2 ] 2 , 1.1 mg (0.4 mol %) of triphenylphosphine and 22.4 mg (20 mol %) of potassium tert-butoxide were added together into a pressure-resistant tube, and after nitrogen replacement, the reaction was carried out at 160° C. for 10 h. The obtained reaction solution was cooled to room temperature, extracted with water and ethyl acetate, the organic phase was separated, dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by rapid preparative liquid chromatography (volume ratio of ethyl acetate: petroleum ether was 30:100). The eluate containing the target compound was collected, and 0.101 g of the compound represented by formula 3 was obtained by rotary evaporation under reduced pressure, with a yield of 45% and a liquid phase...
Embodiment 3
[0031] Example 3: Synthesis of 2,3-diphenylpropanamide
[0032] Benzyl alcohol (0.540 g, 2.0 mmol) and phenylacetonitrile (0.117 g, 1.0 mmol) were dissolved in 5.0 mL of dioxane and mixed with 14.4 mg (5 mol%) of IrCl 3 , 62.2 mg (10.0 mol%) of (±)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, and 28 mg (50 mol%) of potassium hydroxide were added to the pressure-resistant tube After nitrogen replacement, the reaction was carried out at 80 °C for 2 h. The resulting reaction solution was cooled to room temperature, extracted with water and ethyl acetate, the organic phase was separated, dried over anhydrous magnesium sulfate, filtered, concentrated, and purified by rapid preparative liquid chromatography (the volume ratio of ethyl acetate: petroleum ether was 30:100). The eluate containing the target compound was collected, and 0.027 g of the compound represented by formula 3 was obtained by rotary evaporation under reduced pressure, with a yield of 12% and a liquid phase purity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com