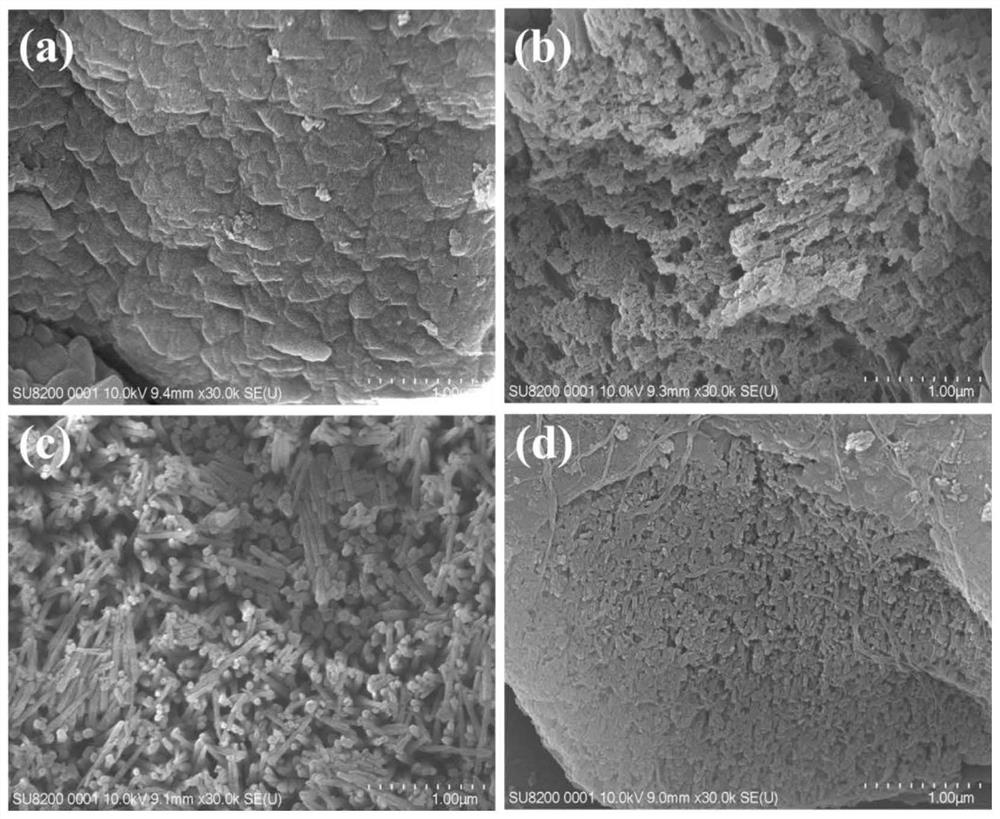
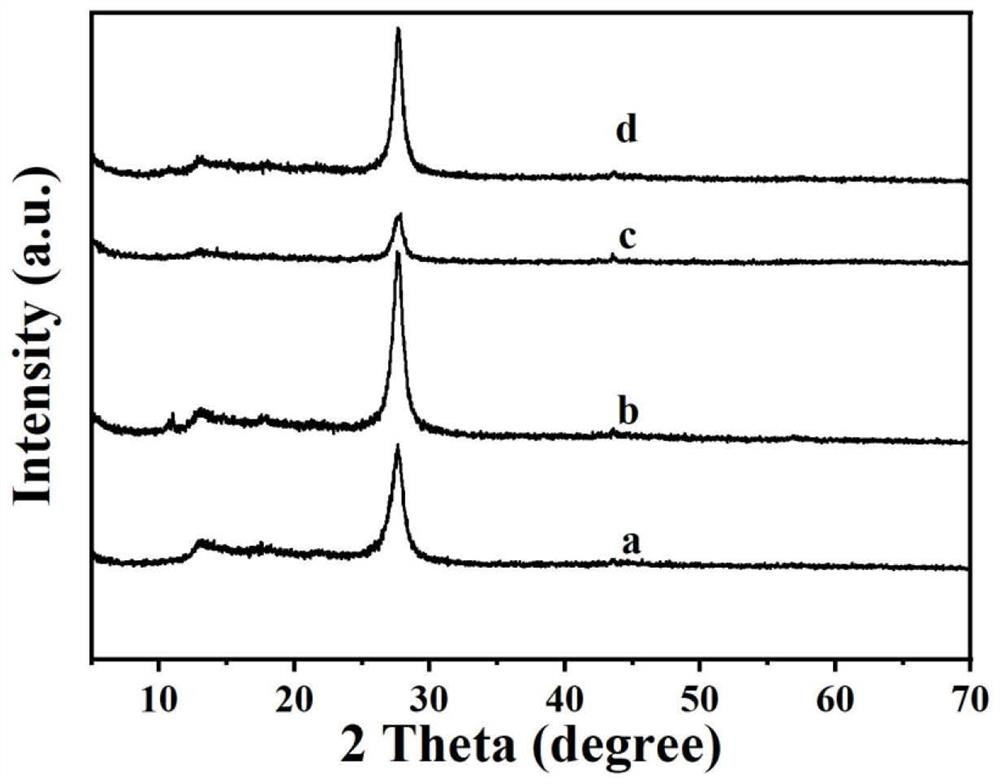
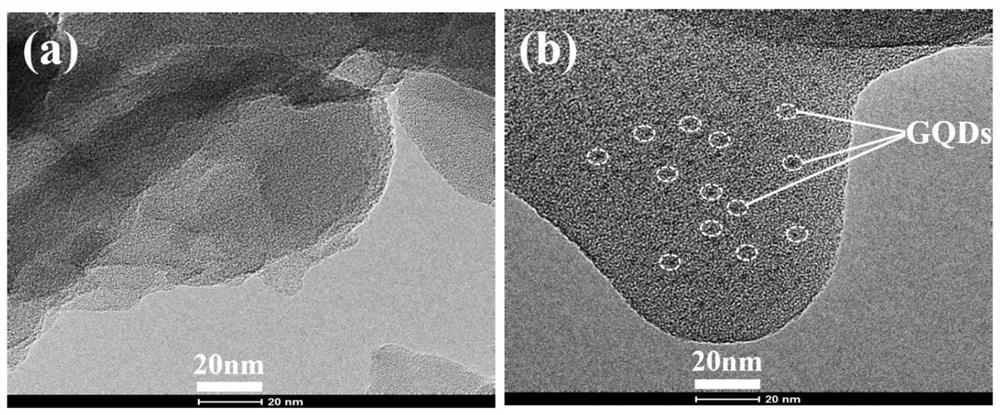
Preparation method of composite visible light catalyst graphene quantum dot/graphite-phase nitrogen carbide
A graphene quantum dot, visible light technology, applied in catalyst activation/preparation, chemical instruments and methods, botanical equipment and methods, etc., can solve problems such as lack of electron hole separation ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] Example 1. Preparation and testing of pure g-C3N4 photocatalyst.
[0029] Step 1: Weigh about 10 g of melamine, place it in a porcelain boat, and calcinate at 550° C. for 4 hours.
[0030] Step 2: After cooling to room temperature, grind the calcined solid into a fine powder with an agate mortar to obtain pure g-C3N4(CN).
[0031] Step 3: Place about 0.08g of catalyst in 10ml of deionized water, ultrasonically disperse for 5 minutes, transfer the suspension to a 10×5×0.1cm3 glass slide with a pipette gun, and dry it in an oven at 60°C.
[0032] Step 4: Place the glass sheet loaded with the photocatalyst in the photocatalytic reactor, and feed a gas flow containing 20ppm NO and 5% O2 with N2 as the balance gas, and the gas flow rate is 200ml / min.
[0033] Step 5: After the gas is introduced, physical adsorption is carried out until the catalyst is saturated with the adsorption of NO in the gas flow. A 300W xenon lamp (λ>380nm) with an ultraviolet light filter is used as...
example 2
[0034] Example 2. Preparation and testing of GQDs / CN composite photocatalysts.
[0035] Step 1: Same as Step 1 and Step 2 of Example 1.
[0036] Step 2: Weigh 2 g of citric acid monohydrate (CA) into a round-bottomed flask, heat in an oil bath at 200°C for 30 min, and CA eventually turns bright orange. The product was slightly cooled, and a certain amount of 0.25 mol / L NaOH was added to adjust the pH of the solution to 7 to obtain a GQDs solution.
[0037] Step 3: Weigh 0.4g of CN in step 1 and mix with 1ml of GQDs solution in step 2, then add high-purity water to reduce the volume of the solution to about 40ml, and stir at room temperature for 30min.
[0038]Step 4: Pour the mixture into a 100ml polytetrafluoroethylene reactor and react at 180°C for 4h.
[0039] Step 5: After cooling the product obtained in step 4 to room temperature, pour the contents of the reactor into a centrifuge tube, centrifuge to remove the supernatant, then wash with deionized water and absolute et...
example 3
[0043] Example 3. Preparation and testing of GQDs / CN composite photocatalysts.
[0044] Step 1: Same as Step 1 in Example 2.
[0045] Step 2: Same as Step 2 in Example 2.
[0046] Step 3: Weigh 0.4g of CN in step 1 and mix with 9ml of GQDs solution in step 2, then add high-purity water to change the volume of the solution to about 40ml, and stir at room temperature for 30min.
[0047] Step 4: Same as Step 4 in Example 2.
[0048] Step 5: Same as Step 5 in Example 2.
[0049] Step 6: Same as Step 3 in Example 1.
[0050] Step 7: Same as Step 4 in Example 1.
[0051] Step 8: Same as Step 5 in Example 1.
[0052] Step 9: Change NO gas to HCHO gas for photocatalytic experiments.
[0053] Step 10: Take a certain amount of catalyst powder for sterilization experiment. The experimental group contains catalyst and is exposed to visible light, and the control group does not contain catalyst and is exposed to visible light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com