Methods and devices for generation of oocytes with improved oocyte quality for in vitro fertilization procedures using non-invasive cellular transfer
A technology of oocytes and mother cells, applied in the direction of epidermal cells/skin cells, non-embryonic pluripotent stem cells, biochemical equipment and methods, etc., to achieve the effect of improving the quality of oocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0126] Effect of Tunneling Nanotube-Forming Cells on Mouse Oocytes Using a Hanging Drop Culture System
[0127] The effect of transferring autologous biomolecules and cellular components to mouse oocytes using the hanging drop method during in vitro maturation was determined. C57 / BL6 mice aged 6 weeks or 12 months were perfused with PMSG (5 IU / female). Female mice were sacrificed 48 hours later by cervical dislocation. Squirt alcohol into the abdomen of the mouse, and make an incision to expose the abdominal cavity. The ovary was dissected with a needle and cut into small pieces. Blastocyst (GV) oocytes were selected and either controlled (medium only) or co-cultured with adult stem cells. Before co-cultivation, cumulus cells around oocytes were removed using 1 mg / μl hyaluronidase in M2 medium (Sigma-Aldrich, USA).
[0128] To mimic poor oocyte quality (such as by inhibiting mitochondrial respiration), oocytes were treated with rotenone at a concentration of 0.05 μΜ for ...
example 2
[0140] Effects of KIF5B overexpression in adult stem cells on mouse oocytes using a hanging drop culture system ring
[0141] The effect of autologous transfer of biomolecules and cellular components from adult stem cells overexpressing the KIF5B gene to mouse oocytes was determined using the hanging drop method. The KIF5B gene was cloned into an expression vector plasmid, and the plasmid was transfected into human adult stem cells.
[0142] C57 / BL6 mice were perfused with PMSG (5 IU / female). Female mice were sacrificed 48 hours later by cervical dislocation. Squirt alcohol into the abdomen of the mouse, and make an incision to expose the abdominal cavity. The ovary was dissected with a needle and cut into small pieces. Blastocyst (GV) oocytes were selected and either controlled (medium only) or co-cultured with adult stem cells. Before co-cultivation, cumulus cells around oocytes were removed using 1 mg / μl hyaluronidase in M2 medium (Sigma-Aldrich, USA). To simulate ...
example 3
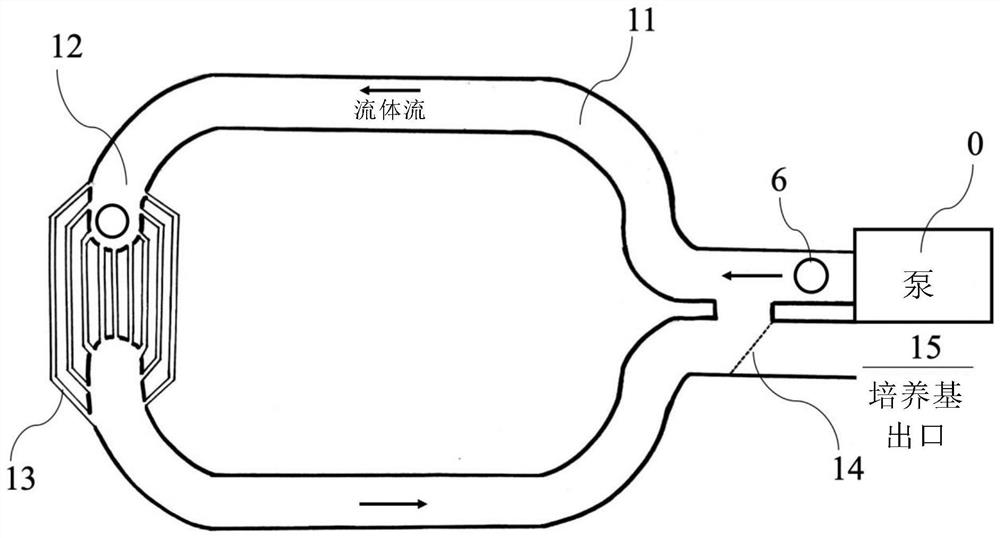
[0149] Effect of adult stem cells on mouse oocytes using the microfluidic device of the present invention
[0150] The effect of using the microfluidic device of the present invention to transfer autologous biomolecules and cellular components from adult stem cells to mouse oocytes was determined in vitro. Six-week-old C57 / BL6 mice were perfused with PMSG (5 IU / female). Female mice were sacrificed 48 hours later by cervical dislocation. Squirt alcohol into the abdomen of the mouse, and make an incision to expose the abdominal cavity. The ovary was dissected with a needle and cut into small pieces. Blastocyst (GV) oocytes were selected and either controlled (medium only) or co-cultured with adult stem cells. Cumulus cells around oocytes were removed using 85 IU / ml hyaluronidase in M2 medium (Sigma-Aldrich, USA) before co-cultivation. To mimic poor oocyte quality (such as by inhibiting mitochondrial respiration), oocytes were treated with rotenone at a concentration of 0.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com