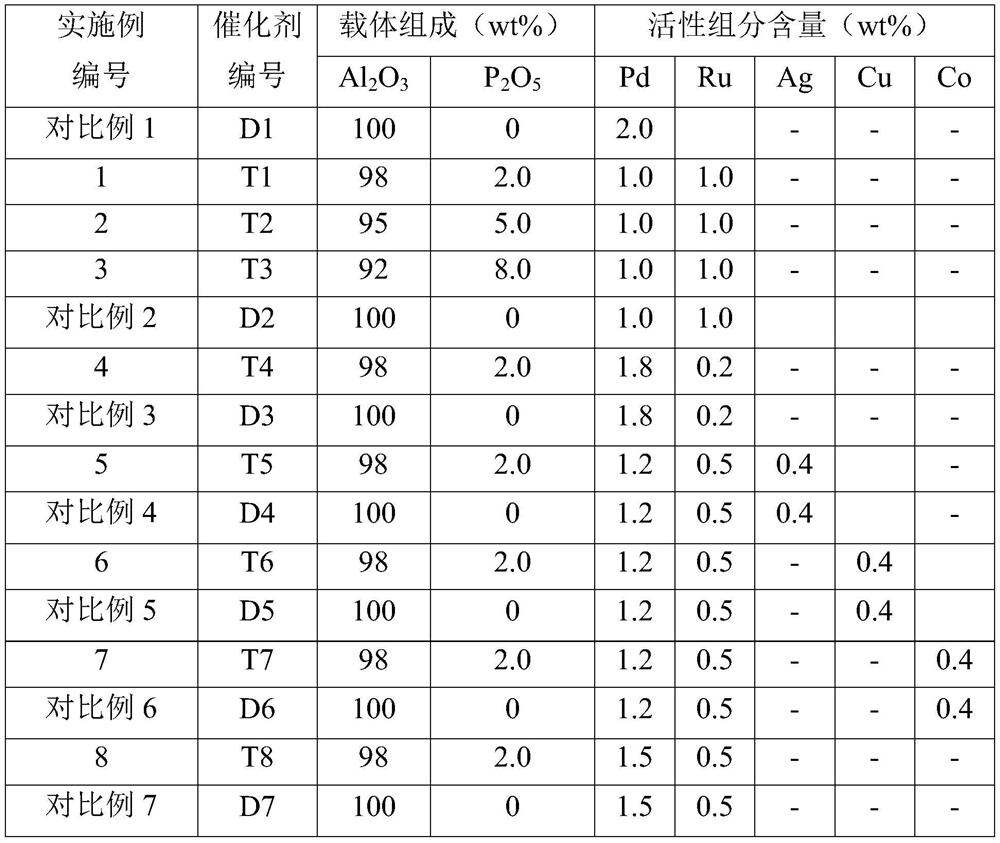
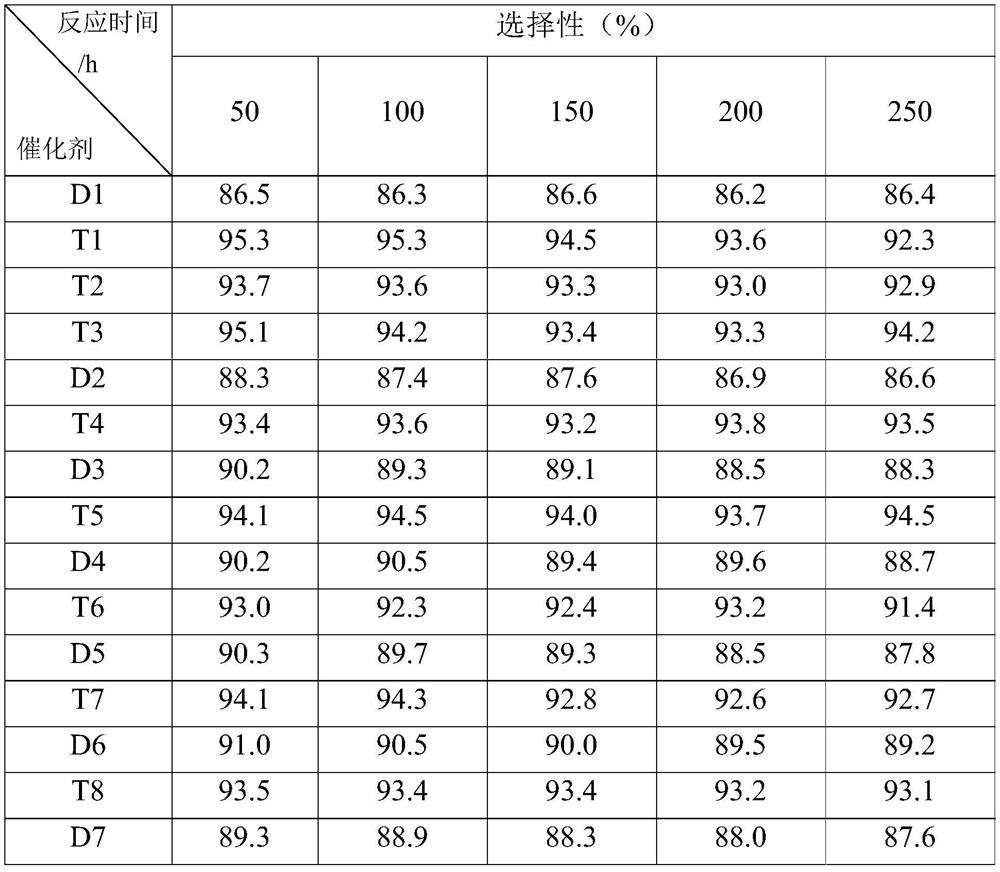
Alkyl anthraquinone hydrogenation method
An alkyl anthracene and hydrogenation catalyst technology, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problems of shortening the service life, affecting the fluidization of the catalyst, affecting the utilization efficiency of the catalyst, etc. The effect of service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The preparation method of hydrogenation catalyst described in the inventive method preferably comprises the following steps:
[0020] a. Dry the activated alumina powder and measure the saturated water absorption;
[0021] b. According to P 2 o 5 / Al 2 o 3 Calculate the P required for 100g of activated alumina with a mass ratio of 0.001 to 0.3 2 o 5 The mass number, then calculate the mass of the corresponding required phosphorus-containing compound;
[0022] c. Weigh the required amount of deionized water and the amount of phosphorus-containing compound, prepare a solution of the corresponding phosphorus-containing compound, fully mix with activated alumina, dry, and roast to obtain the carrier;
[0023] d, impregnating the carrier obtained in step c with a required amount of a palladium-containing compound, a ruthenium-containing compound, and a compound containing a co-active component, and adding NaOH solution dropwise to obtain a catalyst precursor suspension...
Embodiment 1
[0032] (1) carrier preparation
[0033] Weigh 100g γ-Al 2 o 3 (Jiangyan Chemical Auxiliary Factory, Jiangsu Province, with a surface area of 150m 2 / g, the pore volume is 0.4mL / g, the particle size is 30~150μm), the measured saturated water absorption is 70mL, according to P 2 o 5 / Al 2 o 3 Mass ratio 0.02 Weigh 3.25g of ammonium dihydrogen phosphate and add it to 70mL of deionized water required for weighing to prepare the corresponding ammonium phosphate solution, mix it with activated alumina and stir evenly, then move it into an oven and dry at 120°C for 4h. The dried samples were calcined at 550° C. for 8 h to obtain the desired carrier.
[0034] (2) Catalyst preparation
[0035] Measure 100mL palladium chloride and ruthenium chloride concentration to be the aqueous solution of 7.5g / L, 7.5g / L respectively, take the carrier that 75g step (1) obtains, the carrier is dispersed in the above-mentioned solution, immerse 12h at room temperature, drop Add 12.5 mL of 5 (...
Embodiment 2
[0037] (1) carrier preparation
[0038] Weigh 100g γ-Al 2 o 3 (Jiangyan Chemical Auxiliary Factory, Jiangsu Province, with a surface area of 150m 2 / g, the pore volume is 0.4mL / g, the particle size is 30~150μm), the measured saturated water absorption is 70mL, according to P 2 o 5 / Al 2 o 3 Mass ratio 0.05 Weigh 8.2g of ammonium dihydrogen phosphate and add it to 70mL of deionized water required for weighing to prepare the corresponding ammonium phosphate solution, mix it with activated alumina and stir evenly, then move it into an oven and dry at 120°C for 4h. The dried samples were calcined at 550° C. for 8 h to obtain the desired carrier.
[0039] (2) Catalyst preparation
[0040] Measure 100mL palladium chloride and ruthenium chloride concentration to be the aqueous solution of 7.5g / L, 7.5g / L respectively, take the carrier that 75g step (1) obtains, the carrier is dispersed in the above-mentioned solution, immerse 12h at room temperature, drop Add 12.5 mL of 5 (w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com