Production method for coproducing sodium carbonate and ammonium chloride from sodium chloride
A technology of combined preparation and production methods, applied in the directions of ammonium chloride, ammonium halide, carbonate preparations, etc., can solve the problems of high raw water purification requirements, many device components, and high assembly requirements, reducing circulating materials and improving economic benefits. , good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
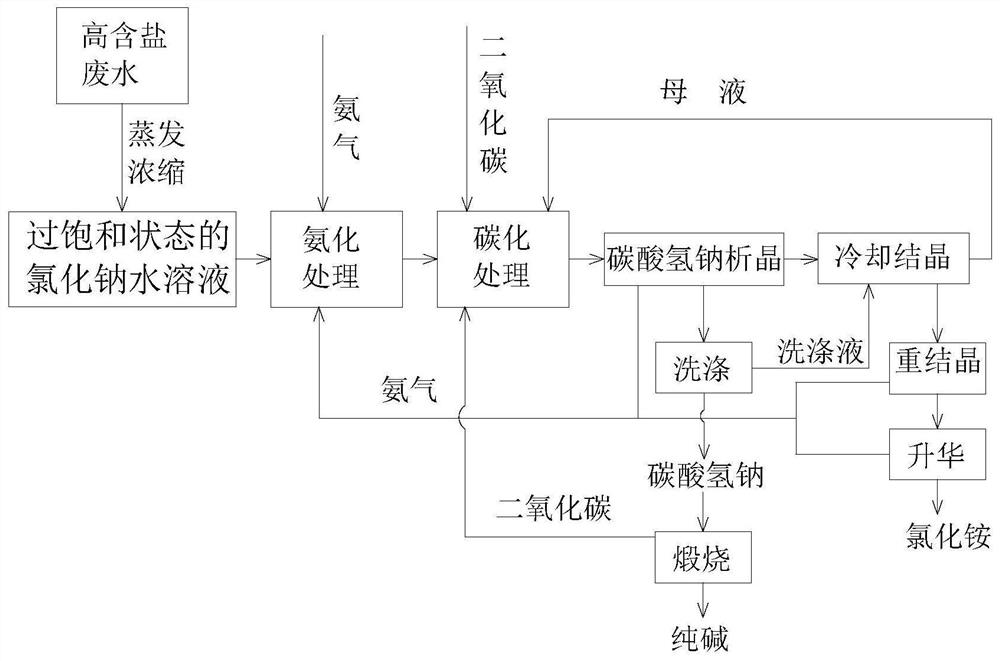
[0026] A method of preparing sodium carbonate with ammonium chloride, including the following steps:
[0027] (1) The high-salt wastewater is evaporated to evaporate, and the aqueous solution of sodium chloride is formed by high pure water ultrasound, wherein the NaCl mass concentration is 30%, and the ammonia gas is continuously passed in the ammonia tower. Absorption, where the molar ratio of ammonia gas and NaCl is 1: 1, the ammonia reaction temperature is 30 ° C, the ammonia time is 0.5 h, the reaction pressure is 0.1 MPa, and the absorption solution is converted to aproductive high salt wastewater.
[0028] (2) Put the ammonification high salt wastewater in step (1) from the pump into the tube, continuously introducing carbon dioxide gas to the carbonization tower, wherein carbon dioxide and NaCl molar ratio of 1.: 1, The carbonized reaction temperature was 20 ° C, the carbonization time was 0.5 h, and the reaction pressure was 0.1 MPa;
[0029] (3) A carbonate salt was forme...
Embodiment 2
[0032] A method of preparing sodium carbonate with ammonium chloride, including the following steps:
[0033] (1) The high-salt wastewater is evaporated to evaporate, and the aqueous solution of sodium chloride is formed into a supersaturated state in a high-pure water ultrasound, wherein the NaCl mass concentration is 40%, and the ammonia gas is continuously passed in the ammonia tower. Absorption, where the molar ratio of ammonia gas and NaCl is 1.5: 1, the ammonia reaction temperature is 40 ° C, the ammonia time is 1 h, the reaction pressure is 0.3 MPa, and the absorption solution is converted to aproductive high salt wastewater.
[0034] (2) Put the ammonium high salt wastewater in step (1) from the pump into the tube, continuously introducing carbon dioxide gas to the carbonization tower, wherein the carbon dioxide and NaCl ratio of 1.3: 1, carbonization The reaction temperature was 35 ° C, the carbonization time was 1 h, and the reaction pressure was 0.3 MPa;
[0035] (3) A ...
Embodiment 3
[0039] A method of preparing sodium carbonate with ammonium chloride, including the following steps:
[0040] (1) The high-salt wastewater is evaporated to evaporate, and the aqueous solution of sodium chloride is formed by high pure water ultrasound, wherein the NaCl mass concentration is 50%, and the ammonia gas is continuously passed into the ammonia. Absorption, where the molar ratio of ammonia gas and NaCl is 2: 1, the ammonia reaction temperature is 50 ° C, the ammonia is 1 h, the reaction pressure is 0.5 MPa, and the absorption solution is converted to ammonium hydrosal water.
[0041](2) The ammonium high salt wastewater in step (1) is flowed into the tower by the pump, and the carbon dioxide gas is continuously introduced into the carbonization tower, wherein the carbon dioxide and NaCl molar ratio are 1.5: 1, carbonization. The reaction temperature was 45 ° C, the carbonization time was 1 h, and the reaction pressure was 0.5 MPa;
[0042] (3) A carbonate salt was formed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com