Quantitative analysis method for escitalopram in human plasma
A quantitative analysis, human plasma technology, applied in the field of biological analysis, can solve the problems of large matrix interference, difficult to obtain samples, low analytes, etc., and achieve the effect of high degree of instrument automation, improved detection sensitivity, and high repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The preparation of embodiment 1 experimental solution and working solution
[0034] Mobile phase A: Measure about 500mL of ultrapure water into a 1L volumetric flask, add 1000μL of formic acid and 2000μL of 2.5M ammonium formate solution, dilute with ultrapure water to volume, ultrasonically degas, and set aside. Storage conditions: room temperature. Validity period: 2 days.
[0035] Mobile phase B: MS grade methanol solution. Storage conditions: room temperature. Validity period: 3 months.
[0036] Methanol: water (30:70, v:v) [denoted as diluent 1]: Add 300mL methanol and 700mL water into a glass bottle, shake and mix. Storage conditions: 4°C. Validity period: 3 months.
[0037] The stock solution of escitalopram was obtained by two independent weighings and prepared respectively. All prepared stock solutions should be stored in a refrigerator at 0-10°C. Before the long-term storage stability data of the stock solution is obtained, it is considered to be stabl...
Embodiment 2
[0055] A LC-MS / MS quantitative analysis method for escitalopram in human plasma, comprising the steps of:
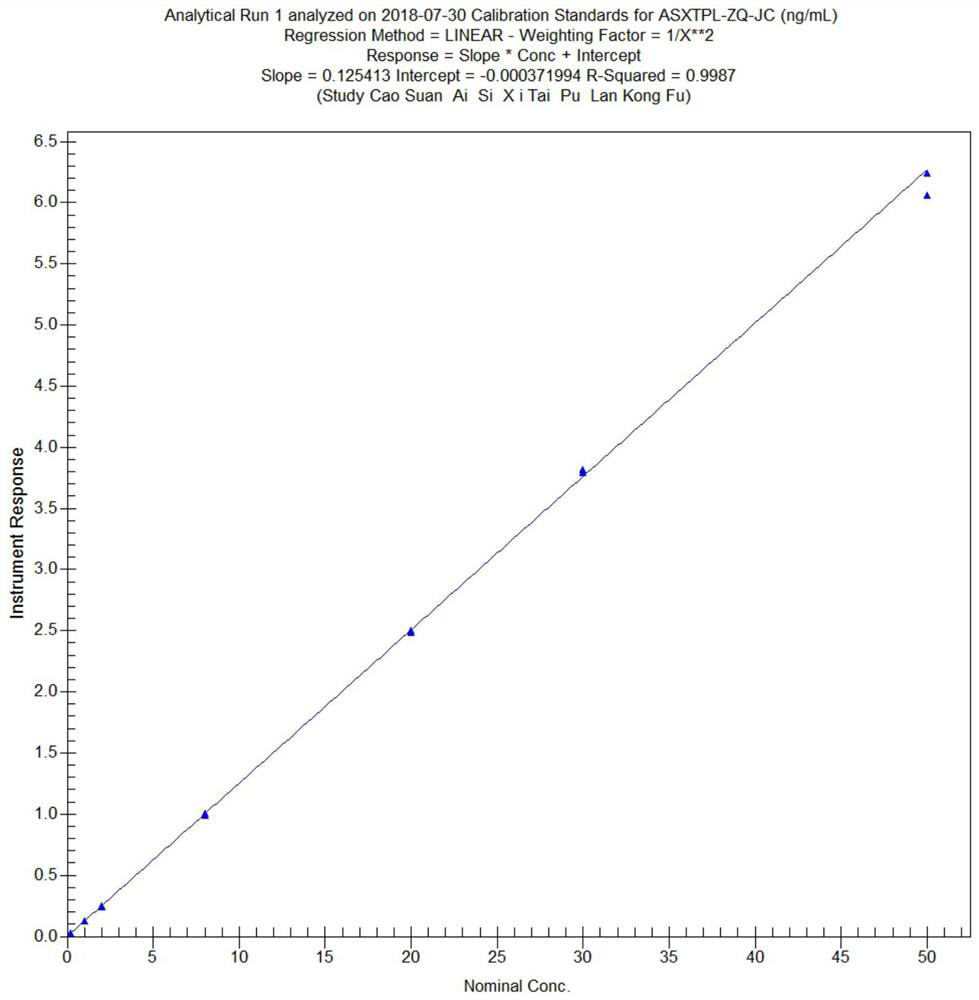
[0056] (1) Preparation of standard working solution: the gradient concentrations of escitalopram working solution are 2.000, 10.00, 20.00, 80.00, 200.0, 300.0, 500.0ng·mL -1 , the concentration gradient of escitalopram quality control working solution is 2.000, 5.000, 100.0, 400.0ng·mL -1 ; The gradient concentrations of the standard curve are 0.1000, 1.000, 2.000, 8.000, 20.00, 30.00, 50.00ng·mL -1 , the concentrations of quality control samples were 0.2000, 0.5000, 10.00, 40.00ng·mL -1 ;
[0057] Internal standard working solution is 100.0ng·mL -1 ;
[0058] The preparation of the above solution only needs to ensure the final concentration, and its preparation method can be based on the preparation method of each solution in Example 1, or can be other methods.
[0059] (2) Sample processing: Using a pipette, add 100.0 μL of blank matrix (blank human plasma), water...
Embodiment 3
[0098] This example evaluates the intra-assay and inter-assay accuracy and precision of the LC-MS / MS method in Example 2 for determining the concentration of escitalopram in human plasma. According to the above method, the quality control samples of the above four concentrations of LLOQQC, LQC, MQC, and HQC were respectively prepared with 6 samples of each concentration, and the measurement of 6 samples of each concentration was repeated 3 times in three consecutive analysis batches. The calculation precision degree, the results are shown in the table below
[0099]
[0100]
[0101]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com