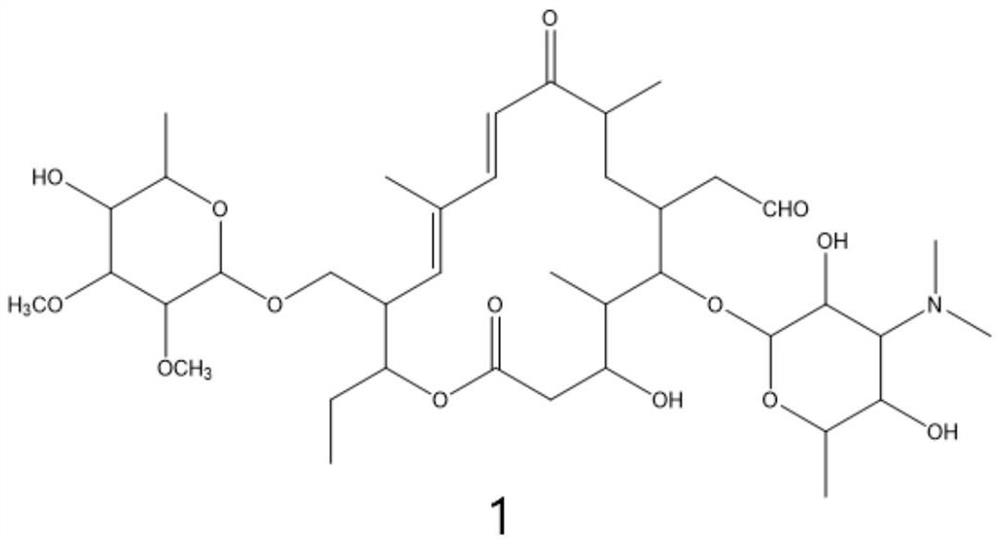
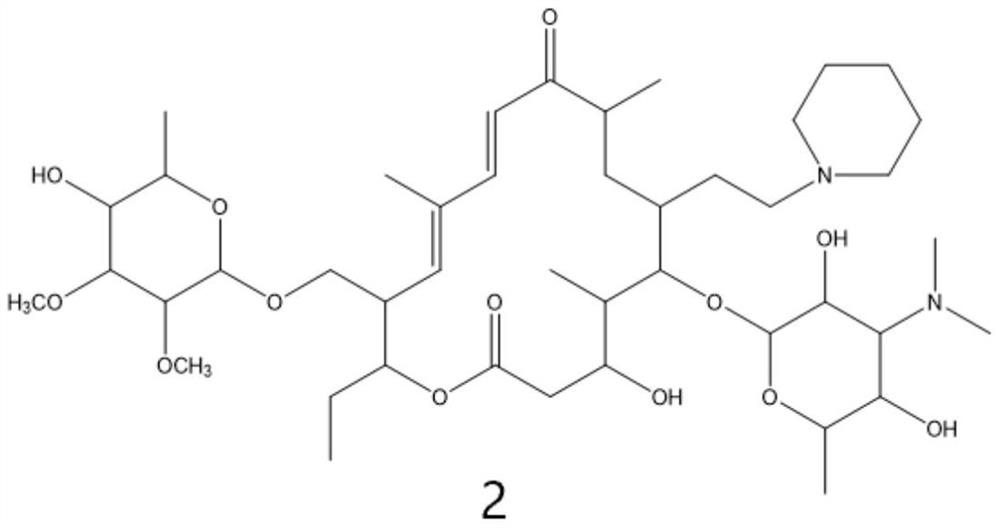
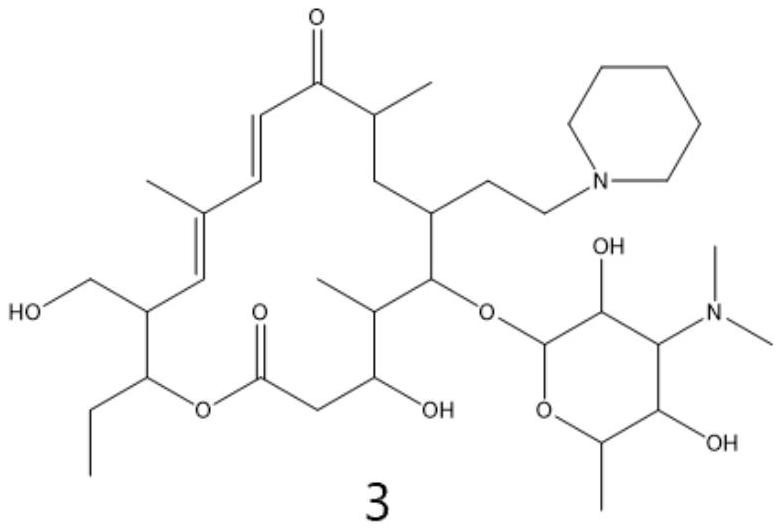
Preparation method of tildipirosin
A technology of tediloxine and tylosin, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc. The effect of few side reactions and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] (1) Primary hydrolysis
[0072] 80 g (0.079 mol) of tylosin phosphate was dissolved in 3000 mL of water, and 40 mL of sulfuric acid with a mass fraction of 20% was added to obtain a reaction solution with a pH value of 2. Heat the reaction solution to 35°C and keep it warm for 1h. After the liquid chromatography monitors the reaction is complete, cool down to room temperature, add 300mL butyl acetate to the reaction solution, add 24mL NaOH solution with a mass fraction of 30% under stirring to adjust the pH value to about 10 , separated after stirring, and the upper organic phase was taken to obtain a primary hydrolyzate. The organic phase directly enters the next reaction.
[0073] (2) Reductive amination
[0074] Add 7.5 g (0.088 mol) of piperidine and 5 g (0.108 mol) of formic acid to the organic phase obtained in (1), insulate and react at 70 ° C for 3 h, add 150 mL of hydrochloric acid solution with a mass fraction of 5% after cooling down to room temperature, an...
Embodiment 2
[0083] (1) Primary hydrolysis
[0084] 80 g (0.079 mol) of tylosin phosphate was dissolved in 3000 mL of water, and 42 mL of sulfuric acid with a mass fraction of 20% was added to obtain a reaction solution with a pH value of 2. Heat the reaction solution to 40°C and keep it warm for 1h. After the liquid chromatography monitors that the reaction is complete, cool down to room temperature, add 300mL of butyl acetate to the reaction solution, add 25mL of 30% NaOH solution under stirring, and adjust the pH value to about 10. After stirring, separate the liquids, take the upper organic phase, and obtain the primary hydrolyzate. The organic phase directly enters the next reaction.
[0085] (2) Reductive amination
[0086] Add 7.6g (0.089mol) of piperidine and 5g (0.108mol) of formic acid to the organic phase obtained in (1), insulate and react at 70°C for 3h, add 150mL mass fraction of 5% hydrochloric acid solution after cooling to room temperature, and adjust the pH value to abo...
Embodiment 3
[0095] (1) Primary hydrolysis
[0096] 81 g (0.079 mol) of tylosin phosphate was dissolved in 3000 mL of water, and 42 mL of sulfuric acid with a mass fraction of 20% was added to obtain a reaction solution with a pH value of 2. Heat the reaction solution to 40°C and keep it warm for 1 hour. After the liquid chromatography monitors that the reaction is complete, cool down to room temperature, add 300mL of butyl acetate to the reaction solution, add 26mL of 30% NaOH solution under stirring, and adjust the pH value to about 11. After stirring, separate the liquids, take the upper organic phase, and obtain the primary hydrolyzate. The organic phase directly enters the next reaction.
[0097] (2) Reductive amination
[0098] Add 7.6g (0.089mol) piperidine and 5g (0.108mol) formic acid to the organic phase obtained in (1), insulate and react at 70°C for 3h, add 150mL mass fraction of 5% hydrochloric acid solution after cooling to room temperature, and adjust the pH value to about...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com