Preparation method of imidic acid or hydrochloride thereof
A technology of imaric acid hydrochloride and imaic acid, applied in the field of preparation of imaric acid or its hydrochloride, can solve the problems of unsuitability for industrial production, large amount of N-methylpiperazine, complicated refining process and the like , to achieve the effect of low cost, high conversion rate and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
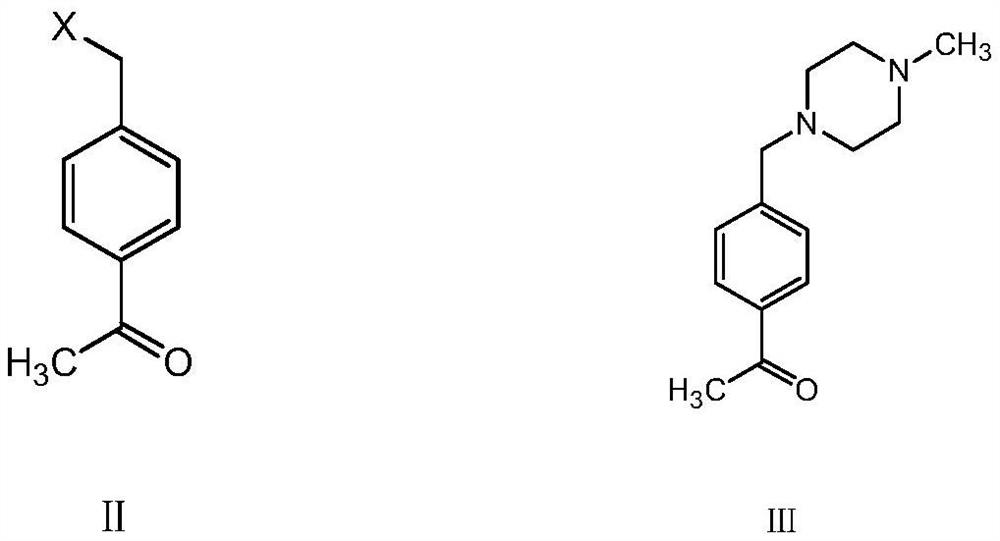
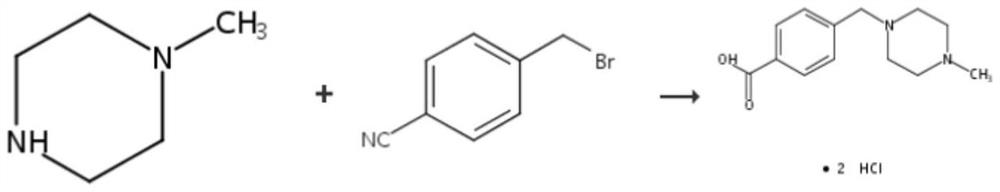
[0043] In a clean reaction flask, add 16.85 g of 4-chloromethyl acetophenone, add 200 mL of ethanol, stir and mix evenly, then add 11 g of N-methylpiperazine and 60 mL of 10% aqueous sodium hydroxide solution in Stir the reaction at 20-30°C for 5 hours, and carry out central control. The HPLC detection purity reaches 99.1%, and the residual 4-chloromethyl acetophenone is 0.15%. After the reaction is completed, the reaction solution is cooled to 0-5°C , slowly drop 300mL of 10% sodium hypochlorite aqueous solution directly into the reaction solution, and control the temperature at 0-5°C for dropwise addition. After the dropwise addition, raise the temperature to 45-55°C for insulation reaction for 3h, central control HPLC The purity of the product was detected to be 99.1%, and the residual intermediate was 0.12%. The temperature was lowered to room temperature, and the solvent was directly removed to obtain the corresponding imaic acid product;
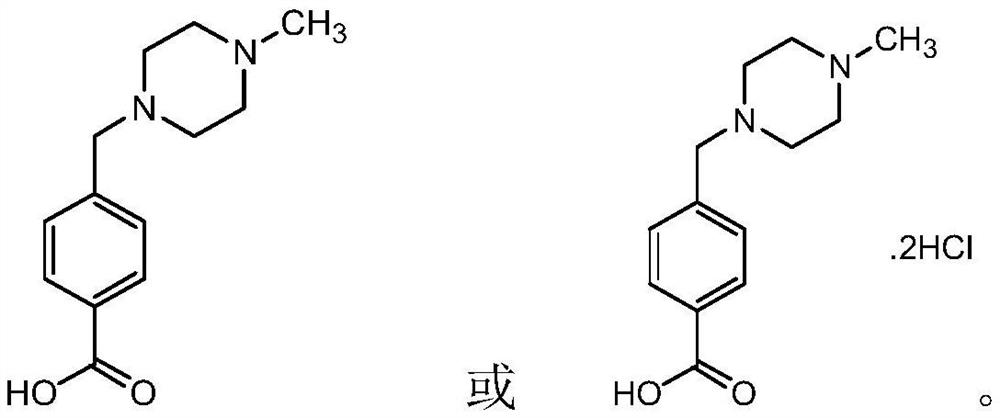
[0044] If you need to synthesiz...
Embodiment 2
[0046] In a clean reaction flask, add 21.3 g of 4-bromomethyl acetophenone, add THF 200 ml, stir and mix evenly, then add 12 g of N-methylpiperazine and 60 ml of 10% aqueous sodium hydroxide solution in After stirring and reacting at 20-30°C for 5 hours, the central control, the HPLC detection purity reached 99.4%, and the residual 4-bromomethylacetophenone was 0.11%. After the reaction, the reaction solution was cooled to 0-5°C, and directly Slowly add 300ml of sodium hypochlorite aqueous solution with a mass percentage of 10% to the reaction solution dropwise, and control the temperature at 0-5°C for dropwise addition. After the dropwise addition, raise the temperature to 45-55°C for insulation reaction for 3 hours, and the central control HPLC detects 99.2 %, the residual intermediate is 0.08%, the temperature is lowered to room temperature, and the solvent is directly removed to obtain the corresponding imaic acid product.
[0047] If you need to synthesize ima hydrochlori...
Embodiment 3
[0049] In a clean reaction flask, add 21.3 g of 4-bromomethyl acetophenone, add 200 ml of methanol, stir and mix evenly, then add 13 g of N-methylpiperazine and 60 ml of 10% aqueous sodium hydroxide solution by mass percentage, After stirring and reacting at 20-30°C for 5 hours, the central control, the HPLC detection purity reached 99.6%, and the residual 4-bromomethyl acetophenone was 0.05%. After the reaction, the reaction solution was cooled to 0-5°C. Slowly add 300ml of sodium hypochlorite aqueous solution with a mass percentage of 10% directly to the reaction solution, and control the temperature at 0-5°C for dropwise addition. After the dropwise addition, raise the temperature to 45-55°C for insulation reaction for 3 hours, and central control HPLC detection 99.7%, intermediate residual 0.04%, cooled to room temperature, directly distilled off the solvent to obtain the corresponding imaic acid product.
[0050] If you need to synthesize ima hydrochloride, you can add co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com