Biomass derivative methyl furan-2,5-dimethyl carbamate and preparation method thereof
A technology of methyl dimethyl carbamate and derivatives, which is applied in the field of biomass derivatives - methyl furan 2,5-dimethyl carbamate and its preparation, which can solve the problem of polluted environment, severe corrosion of equipment, and industrial applications Limitation and other issues, to achieve the effect of mild reaction conditions, high yield, and easy control of the process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
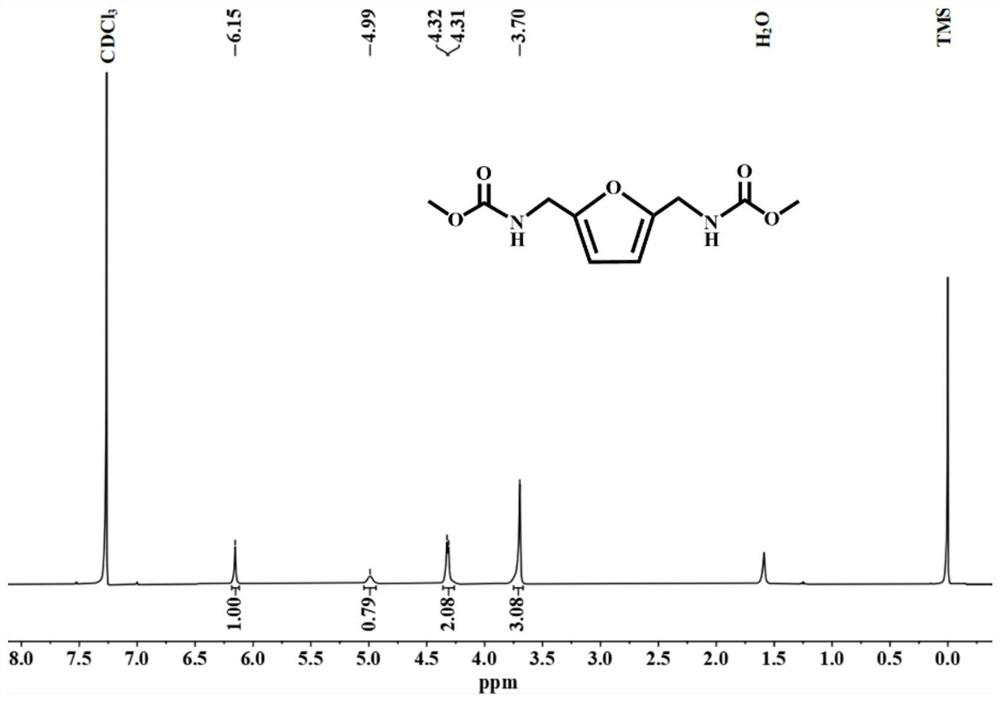
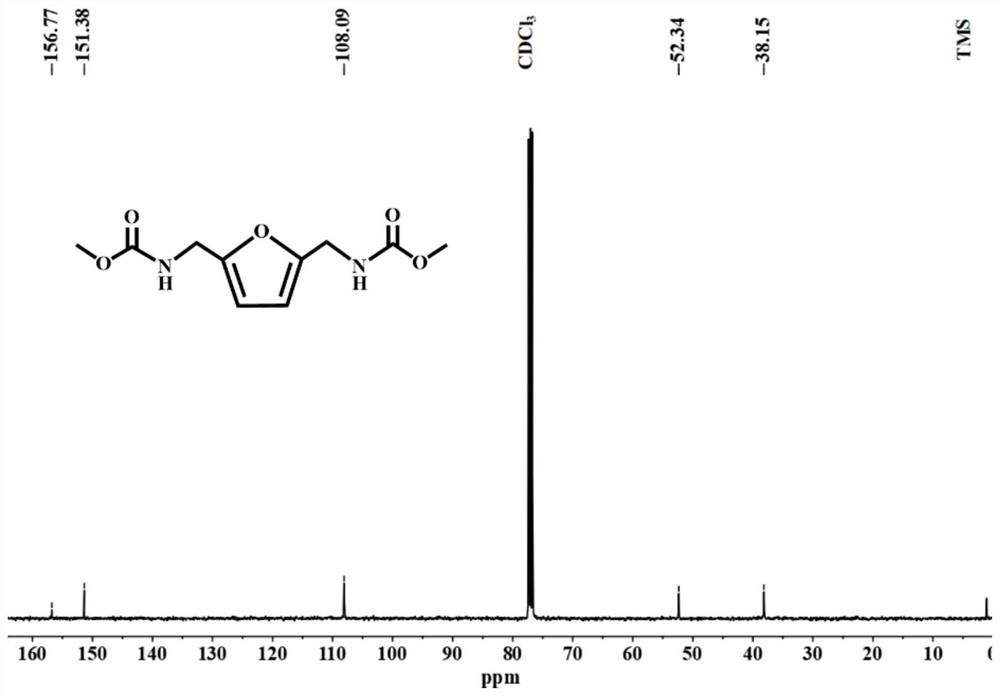
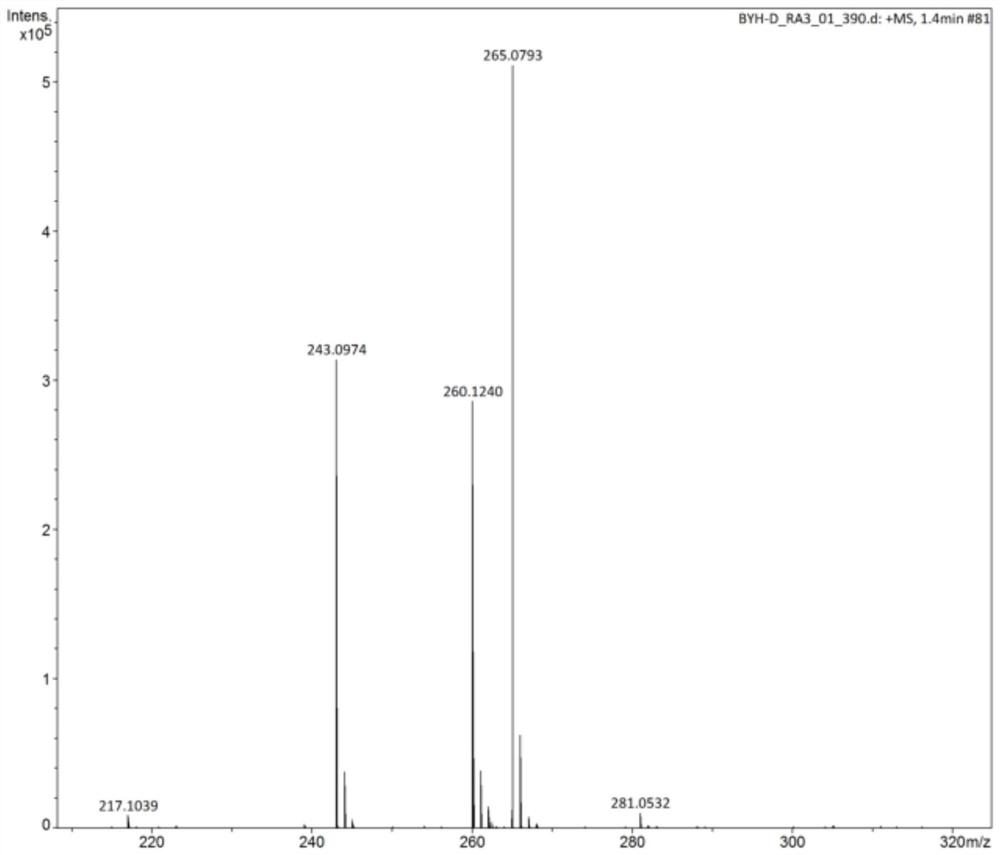
[0026] Under a nitrogen atmosphere, 1.56 g (ie 0.012 mol) of 2,5-bis(aminomethyl)furan, 30 ml (ie 0.35 mol) of dimethyl carbonate and 0.25 g of sodium methoxide were added to a Schlenk tube. Then, the mixture was heated to 85° C. and refluxed for 4 hours under stirring, and then the heating was stopped. After the temperature dropped to room temperature, 17wt% hydrochloric acid solution was added dropwise to the reaction solution to pickle to pH=1-2, then filtered, and at the same time washed with distilled water until neutral, and finally recrystallized by absolute ethanol to obtain white crystals. According to high-performance liquid chromatography analysis, the conversion rate of 2,5-bis(aminomethyl)furan was 100%, the yield of methyl furan-2,5-dimethylcarbamate was 99%, and the purity was 99.99%.
[0027] The structural formula of the new compound furan-2,5-dimethylcarbamate methyl ester provided in this example is as follows:
[0028]
[0029] The above-mentioned compo...
Embodiment 2
[0041] Under a nitrogen atmosphere, 1.56 g (ie 0.012 mol) of 2,5-bis(aminomethyl)furan, 30 ml (ie 0.35 mol) of dimethyl carbonate and 1.10 g of sodium methoxide were added to a Schlenk tube. Then, the mixture was heated to 65° C. and refluxed for 6 hours under stirring, and then the heating was stopped. After the temperature dropped to room temperature, 17wt% hydrochloric acid solution was added dropwise to the reaction solution to pickle to pH=1-2, then filtered, and at the same time washed with distilled water until neutral, and finally recrystallized by absolute ethanol to obtain white crystals. According to high-performance liquid chromatography analysis, the conversion rate of 2,5-bis(aminomethyl)furan was 100%, the yield of methyl furan-2,5-dimethylcarbamate was 98%, and the purity was 99.99%.
Embodiment 3
[0043]1.56g (that is, 0.012 moles) of 2,5-bis(aminomethyl)furan, 30ml (that is, 0.35 moles) of dimethyl carbonate and 0.25g of zinc acetate were added to the autoclave, purged with nitrogen, and then stirred Under the conditions, heat up to 160° C. and react for 7 hours, then stop heating. When the temperature dropped to room temperature, non-condensable gas was released, heated to dissolve the precipitated solid, and then vacuum filtered to remove the solid catalyst while it was hot. Then, the solid obtained after the filtrate was distilled under reduced pressure was recrystallized with absolute ethanol to obtain white crystals. According to high-performance liquid chromatography analysis, the conversion rate of 2,5-bis(aminomethyl)furan was 100%, the yield of methyl furan-2,5-dimethylcarbamate was 95%, and the purity was 99.99%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting range | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com